Medical term:
Depakote
valproate sodium
valproic acid
divalproex sodium
Pharmacologic class: Carboxylic acid derivative
Therapeutic class: Anticonvulsant, mood stabilizer, antimigraine agent
Pregnancy risk category D
Action
Increases level of gamma-aminobutyric acid in brain, reducing seizure activity
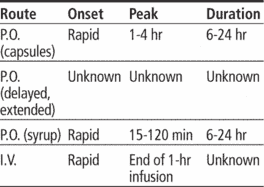
Availability
valproate sodium
Injection: 100 mg/ml in 5-ml vial
Syrup: 250 mg/5 ml
valproic acid
Capsules (delayed-release): 125 mg, 250 mg, 500 mg
Capsules (liquid-filled): 250 mg
divalproex sodium
Capsules (containing coated particles or sprinkles): 125 mg
Tablets (enteric-coated, delayed-release): 125 mg, 250 mg, 500 mg
Tablets (extended-release): 250 mg, 500 mg
Indications and dosages
➣ Complex partial seizures
Adults and children older than age 10: Initially, 10 to 15 mg/kg/day P.O. or I.V. (valproate sodium). May increase by 5 to 10 mg/kg/day q week until blood drug level is 50 to 100 mcg/ml or adverse reactions occur; don't exceed 60 mg/kg/day. If daily dosage exceeds 250 mg, give in two divided doses.
➣ Simple or complex absence seizures
Adults and children older than age 10: Initially, 15 mg/kg/day P.O. or I.V. (valproate sodium). May increase by 5 to 10 mg/kg/day at weekly intervals until therapeutic blood drug level is reached or adverse reactions occur; don't exceed 60 mg/kg/day. If daily dosage exceeds 250 mg, give in two divided doses.
➣ Mania associated with bipolar disorder
Adults: Initially, 750 mg (divalproex or valproic acid delayed-release) P.O. daily in divided doses. Titrate rapidly to desired effect or trough level of 50 to 125 mcg/ml. Don't exceed 60 mg/kg/day.
➣ To prevent migraine
Adults: 250 mg (divalproex or valproic acid delayed-release) P.O. b.i.d. Or 500 mg (divalproex extended-release) P.O. daily for 1 week (up to 1 g/day). Maximum dosage is 1 g/day.
Off-label uses
• Chorea
• Photosensitivity-related seizures
• Sedative-hypnotic withdrawal
Contraindications
• Hypersensitivity to drug or tartrazine (some products)
• Hepatic impairment
• Urea cycle disorders
Precautions
Use cautiously in:
• bleeding disorders, organic brain disease, bone marrow depression, renal impairment
• posttraumatic seizures caused by head injury (use not recommended)
• history of hepatic disease
• elderly patients
• pregnant or breastfeeding patients
• children.
Administration
• Give I.V. only when oral therapy isn't feasible.
• For I.V. use, dilute valproate sodium in at least 50 ml of dextrose 5% in water, lactated Ringer's solution, or normal saline solution. Infuse over 1 hour at a rate slower than 20 mg/minute.
• Know that I.V. and P.O. dosages and dosing frequencies are identical. However, patient should be switched to oral therapy as soon as possible.
• Give oral forms with food.
• Be aware that divalproex extended-release and delayed-release forms are not bioequivalent.
• Make sure patient swallows divalproex extended-release tablets and valproic acid capsules whole without chewing or crushing.
• If patient can't swallow capsule containing coated particles, sprinkle entire contents of capsule onto about 5 ml of semisolid food, such as pudding or applesauce, immediately before giving.
• Don't give syrup in carbonated beverages (may cause mouth and throat irritation).
Adverse reactions
CNS: confusion, dizziness, headache, sedation, ataxia, paresthesia, asthenia, tremor, drowsiness, emotional lability, abnormal thinking, amnesia, hyperammonemic encephalopathy, suicidal behavior or ideation
EENT: amblyopia, blurred vision, nystagmus, tinnitus, pharyngitis
GI: nausea, vomiting, diarrhea, abdominal pain, dyspepsia, anorexia, pancreatitis
Hematologic: leukopenia, thrombocytopenia
Hepatic: hepatotoxicity
Metabolic: hyperammonemia
Musculoskeletal: back pain
Respiratory: dyspnea
Skin: rash, alopecia, bruising
Other: abnormal taste, increased appetite, weight gain, flulike symptoms, infection, infusion site pain and reaction, multiorgan hypersensitivity reaction
Interactions
Drug-drug. Activated charcoal, cholestyramine: decreased valproate absorption
Antiplatelet agents (including abciximab, aspirin and other nonsteroidal anti-inflammatory drugs, eptifibatide, tirofiban), cefamandole, cefoperazone, cefotetan, heparin, thrombolytics, warfarin: increased risk of bleeding
Barbiturates, primidone: decreased metabolism and greater risk of toxicity of these drugs, decreased valproate efficacy
Carbamazepine: increased carbamazepine blood level, decreased valproate blood level, poor seizure control
Chlorpromazine: decreased valproate clearance and increased trough level
Cimetidine: decreased valproate clearance
Clonazepam: absence seizures in patients with history of these seizures
CNS depressants (such as antihistamines and antidepressants, MAO inhibitors, opioid analgesics, sedative- hypnotics): additive CNS depression
Diazepam: displacement of diazepam from binding site, inhibited diazepam metabolism
Erythromycin, felbamate: increased valproate blood level, greater risk of toxicity
Ethosuximide: inhibited ethosuximide metabolism
Lamotrigine: decreased valproate blood level, increased lamotrigine blood level
Phenytoin: increased phenytoin effects and risk of toxicity, decreased valproate effects
Salicylates (large doses in children): increased valproate effects
Topiramate: increased risk of hyperammonemia with and without encephalopathy and hypothermia
Tricyclic antidepressants: increased blood levels of these drugs, greater risk of adverse reactions
Zidovudine: decreased zidovudine clearance in patients with human immunodeficiency virus
Drug-diagnostic tests. Alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, bilirubin: increased levels
Bleeding time: prolonged
Ketone bodies: false-positive results
Platelets, white blood cells: decreased counts
Thyroid function tests: interference with results
Drug-behaviors. Alcohol use: additive CNS depression
Patient monitoring
Closely monitor neurologic status. Watch for seizures and suicidal behavior or ideation.
If hyperammonemia or hyperammonemic encephalopathy (unexplained lethargy and vomiting or changes in mental status) is suspected, measure ammonia level.
Evaluate GI status. Stay alert for signs and symptoms of pancreatitis. Consider discontinuing drug if pancreatitis is diagnosed.
Watch for diverse signs and symptoms of multiorgan hypersensitivity reaction, such as fever and rash associated with other organ system involvement (lymphadenopathy, hepatitis, liver function test abnormalities, hematologic abnormalities, pruritus, nephritis, oliguria, hepatorenal syndrome, arthralgia, and asthenia). Discontinue drug if multiorgan hypersensitivity reaction occurs.
• Monitor I.V. infusion site for local reactions.
• Assess CBC (including platelet count), prothrombin time, International Normalized Ratio, and liver function tests.
• Monitor valproate blood level; therapeutic range is 50 to 100 mcg/ml.
Patient teaching
• Instruct patient to take with food to minimize GI upset.
• Tell patient taking extended-release tablets or valproic acid capsules to swallow them whole without chewing or breaking.
• Inform patient taking capsules with delayed-release pellets that he may swallow them whole or open them and sprinkle contents onto a teaspoon of semisolid food, such as pudding or applesauce.
• Tell patient (or parents) that valproate syrup shouldn't be taken with carbonated beverages.
Advise patient to immediately report signs and symptoms of liver dysfunction (such as malaise, weakness, lethargy, appetite loss, vomiting, or yellowing of skin or eyes), signs and symptoms of pancreatitis (such as abdominal pain, nausea, vomiting, loss of appetite), or suicidal behavior or ideation.
Tell patient to immediately report unexplained signs and symptoms that may reflect hypersensitivity reaction (fever, rash, hepatitis signs and symptoms, bleeding or bruising, itching, urinary problems, muscle pains, or weakness).
• If patient is taking drug for seizure control, tell him to avoid driving and other hazardous activities.
Caution patient not to stop therapy abruptly.
• Instruct patient to avoid alcohol.
• Stress importance of follow-up laboratory tests.
• As appropriate, review all other significant and life-threatening adverse reactions and interactions, especially those related to the drugs, tests, and behaviors mentioned above.
Depakote
(dĕp′ə-kōt′)Patient discussion about Depakote
Q. My brother has had serious side effect with depakote like stomach cramps.. My brother has had serious side effect with depakote like stomach cramps, hair loss, nausea and indigestion when he had jaundice and later the doctor changed the med to lamotrigine ….will this have any side effect….?
Q. Will my bipolar meds (lamictal and depakote) interfere with my birth control pills? We have been married for the past 12 yrs but we don’t have a child because I am paranoid of delivery. But it doesn’t affect our intimacy. I am using birth control pills for the past few years and I could rely on it. Now the new problem is that I was recently diagnosed as bipolar-II. The Doctor prescribed some medicines for me. Will my bipolar meds (Lamictal and Depakote) interfere with my birth control pills?
Latest Searches:
Voraxaze - Voranil - Voorhoeve - voodoo - VOO - Vontrol - von - vomitus - vomiturition - vomitory - vomitoria - vomito - vomitive - vomiting - vomit - vomica - vomerovaginalis - vomerovaginal - vomerorostralis - vomerorostral -
- Service manuals - MBI Corp