Medical term:
Lanoxin
Lanoxin
[lah-nok´sin]digoxin
Pharmacologic class: Cardiac glycoside
Therapeutic class: Inotropic, antiarrhythmic
Pregnancy risk category C
Action
Increases force and velocity of myocardial contraction and prolongs refractory period of atrioventricular (AV) node by increasing calcium entry into myocardial cells. Slows conduction through sinoatrial and AV nodes and produces antiarrhythmic effect.
Availability
Oral solution (pediatric): 0.05 mg/ml
Injection: 0.05 mg/ml, 0.1 mg/ml, 0.25 mg/ml
Tablets: 0.125 mg, 0.25 mg, 0.5 mg
Indications and dosages
➣ Heart failure; tachyarrhythmias; atrial fibrillation and flutter; paroxysmal atrial tachycardia
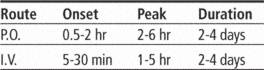
Adults: For rapid digitalizing, 0.6 to 1 mg I.V. over 24 hours, with 50% of total dosage given initially and additional fractions given at 4- to 8-hour intervals; or digitalizing dose of 0.75 to 1.25 mg P.O. over 24 hours, with 50% of total dosage given initially and additional fractions given at 4- to 8-hour intervals. Maintenance dosage is 0.063 to 0.5 mg/day (tablets) or 0.35 to 0.5 mg/day (gelatin capsules), depending on lean body weight, renal function, and drug blood level.
Children older than age 10: For rapid digitalizing, 8 to 12 mcg/kg I.V. over 24 hours, with 50% of total dosage given initially and additional fractions given at 4- to 8-hour intervals; or digitalizing dose of 10 to 15 mcg/kg P.O. over 24 hours, with 50% of total dosage given initially and additional fractions given at 6- to 8-hour intervals. Maintenance dosage is 25% to 35% of loading dosage, given daily as a single dose (determined by renal function).
Children ages 5 to 10: For rapid digitalizing, 15 to 30 mcg/kg I.V. over 24 hours, with 50% of total dosage given initially and additional fractions given at 4- to 8-hour intervals; or digitalizing dose of 20 to 35 mcg/kg P.O. over 24 hours, with 50% of total dosage given initially and additional fractions given at 6- to 8-hour intervals. Maintenance dosage is 25% to 35% of loading dosage, given daily in two divided doses (determined by renal function).
Children ages 2 to 5: For rapid digitalizing, 25 to 35 mcg/kg I.V. over 24 hours, with 50% of total dosage given initially and additional fractions given at 4- to 8-hour intervals; or digitalizing dose of 30 to 40 mcg/kg P.O. over 24 hours, with 50% of total dosage given initially and additional fractions given at 6- to 8-hour intervals. Maintenance dosage is 25% to 35% of loading dosage, given daily in two divided doses (determined by renal function).
Children ages 1 to 24 months: For rapid digitalizing, 30 to 50 mcg/kg I.V. over 24 hours, with 50% of total dosage given initially and additional fractions given at 4- to 8-hour intervals; or digitalizing dose of 35 to 60 mcg/kg P.O. over 24 hours, with 50% of total dosage given initially and additional fractions given at 6- to 8-hour intervals. Maintenance dosage is 25% to 35% of loading dosage, given daily in two divided doses (determined by renal function).
Infants (full-term): For rapid digitalizing, 20 to 30 mcg/kg I.V. over 24 hours, with 50% of total dosage given initially and additional fractions given at 4- to 8-hour intervals; or digitalizing dose of 25 to 35 mcg/kg P.O. over 24 hours, with 50% of total dosage given initially and additional fractions given at 6- to 8-hour intervals. Maintenance dosage is 25% to 35% of loading dosage, given daily in two divided doses (determined by renal function).
Infants (premature): For rapid digitalizing, 15 to 25 mcg/kg I.V. over 24 hours, with 50% of total dosage given initially and additional fractions given at 4- to 8-hour intervals; or digitalizing dose of 20 to 30 mcg/kg P.O. over 24 hours, with 50% of total dosage given initially and additional fractions given at 6- to 8-hour intervals. Maintenance dosage is 20% to 30% of loading dosage, given daily in two divided doses (determined by renal function).
Dosage adjustment
• Renal impairment
• Hyperthyroidism
• Elderly patients
Off-label uses
• Supraventricular tachyarrhythmias
• Intrauterine tachyarrhythmias
Contraindications
• Hypersensitivity to drug
• Uncontrolled ventricular arrhythmias
• AV block
• Idiopathic hypertrophic subaortic stenosis
• Constrictive pericarditis
Precautions
Use cautiously in:
• renal or hepatic impairment, electrolyte imbalances, myocardial infarction, thyroid disorders
• obesity
• elderly patients
• pregnant or breastfeeding patients.
Administration
• Administer I.V. drug undiluted, or dilute with sterile water for injection, normal saline solution, or dextrose 5% in water as directed.
☞ Know that drug has narrow therapeutic index, so dosage must be monitored regularly and patient must be monitored for signs and symptoms of toxicity.
• Know that for rapid effect, initial digitalizing dose generally is given in several divided doses over 12 to 24 hours.
• Be aware that dosages used for atrial arrhythmias generally are higher than those used for inotropic effect.
Adverse reactions
CNS: fatigue, headache, asthenia
CV: bradycardia, ECG changes, arrhythmias
EENT: blurred or yellow vision
GI: nausea, vomiting, diarrhea
GU: gynecomastia
Hematologic: thrombocytopenia
Other: decreased appetite
Interactions
Drug-drug. Amiodarone, cyclosporine, diclofenac, diltiazem, propafenone, quinidine, quinine, verapamil: increased digoxin blood level, possibly leading to toxicity
Amphotericin B, corticosteroids, mezlocillin, piperacillin, thiazide and loop diuretics, ticarcillin: hypokalemia, increased risk of digoxin toxicity
Antacids, cholestyramine, colestipol, kaolin/pectin: decreased digoxin absorption
Beta-adrenergic blockers, other antiarrhythmics (including disopyramide, quinidine): additive bradycardia
Laxatives (excessive use): hypokalemia, increased risk of digoxin toxicity
Spironolactone: reduced digoxin clearance, increased risk of digoxin toxicity
Thyroid hormones: decreased digoxin efficacy
Drug-diagnostic tests. Creatine kinase: increased level
Drug-food. High-fiber meal: decreased digoxin absorption
Drug-herbs. Coca seed, coffee seed, cola seed, guarana seed, horsetail, licorice, natural stimulants (such as aloe), yerba maté: increased risk of digoxin toxicity and hypokalemia
Ephedra (ma huang): arrhythmias
Hawthorn: increased risk of adverse cardiovascular effects
Indian snakeroot: bradycardia
Psyllium: decreased digoxin absorption
St. John's wort: decreased blood level and effects of digoxin
Patient monitoring
• Assess apical pulse regularly for 1 full minute. If rate is less than 60 beats/minute, withhold dose and notify prescriber.
☞ Monitor for signs and symptoms of drug toxicity (such as nausea, vomiting, visual disturbances, arrhythmias, and altered mental status). Be aware that therapeutic digoxin levels range from 0.5 to 2 ng/ml.
• Monitor ECG and blood levels of digoxin, potassium, magnesium, calcium, and creatinine.
• Stay alert for hypocalcemia. Know that this condition may predispose patient to digoxin toxicity and may decrease digoxin efficacy.
☞ Watch closely for hypokalemia and hypomagnesemia. Know that digoxin toxicity may occur with these conditions despite digoxin blood levels below 2 ng/ml.
Patient teaching
• Tell patient to take drug at same time every day.
☞ Instruct patient not to stop drug abruptly.
• Instruct patient not to take over-the-counter drugs without prescriber's approval.
☞ Teach patient how to recognize and report signs and symptoms of digoxin toxicity.
• Stress importance of follow-up testing as directed by prescriber.
• As appropriate, review all other significant and life-threatening adverse reactions and interactions, especially those related to the drugs, tests, foods, and herbs mentioned above.
Lanoxin
(lə-nŏk′sĭn)Lanoxin®
Digoxin, see there.Lanoxin
A brand name for DIGOXIN.Latest Searches:
Voraxaze - Voranil - Voorhoeve - voodoo - VOO - Vontrol - von - vomitus - vomiturition - vomitory - vomitoria - vomito - vomitive - vomiting - vomit - vomica - vomerovaginalis - vomerovaginal - vomerorostralis - vomerorostral -
- Service manuals - MBI Corp