Medical term:
Maxeran
metoclopramide hydrochloride
Pharmacologic class: Dopamine antagonist
Therapeutic class: Antiemetic, GI stimulant
Pregnancy risk category B
FDA Box Warning
• Drug can cause tardive dyskinesia, a serious movement disorder that's often irreversible. Risk of developing tardive dyskinesia increases with duration of treatment and total cumulative dose.
• Discontinue drug in patients who develop signs or symptoms of tardive dyskinesia, which has no known treatment. In some patients, signs and symptoms may lessen or resolve after drug is stopped.
• Avoid using metoclopramide for longer than 12 weeks except in rare cases in which potential benefit outweighs risk of developing tardive dyskinesia.
Action
Blocks dopamine receptors by disrupting CNS chemoreceptor trigger zone, increasing peristalsis and promoting gastric emptying
Availability
Injection: 5 mg/ml
Solution: 5 mg/5 ml
Solution (concentrated): 10 mg/ml
Tablets: 5 mg, 10 mg
Tablets (orally disintegrating): 5 mg, 10 mg
Indications and dosages
➣ To prevent chemotherapy-induced vomiting
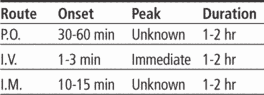
Adults: 1 to 2 mg/kg I.V. 30 minutes before chemotherapy, then q 2 hours for two doses, then q 3 hours for three additional doses
➣ To facilitate small-bowel intubation; radiologic examination when delayed gastric emptying interferes
Adults and children older than age 14: 10 mg I.V. as a single dose
Children ages 6 to 14: 2.5 to 5 mg I.V. as a single dose
Children younger than age 6: 0.1 mg/kg I.V. as a single dose
➣ Diabetic gastroparesis
Adults: 10 mg P.O. 30 minutes before meals and at bedtime for 2 to 8 weeks. If patient can't tolerate P.O. doses, give same dosage I.V. or I.M.
➣ Gastroesophageal reflux
Adults: 10 to 15 mg P.O. 30 minutes before meals and at bedtime for up to 12 weeks. For prevention, single dose of 20 mg (some patients may respond to doses as small as 5 mg).
➣ Prevention of postoperative nausea and vomiting
Adults: 10 to 20 mg I.M. near end of surgical procedure. Repeat dose q 4 to 6 hours, as needed.
Dosage adjustment
• Renal impairment
Off-label uses
• Hiccups
Contraindications
• Hypersensitivity to drug
• Pheochromocytoma
• Parkinson's disease
• Suspected GI obstruction, perforation, or hemorrhage
• History of seizure disorders
Precautions
Use cautiously in:
• diabetes mellitus, renal dysfunction
• history of depression
• elderly patients
• pregnant or breastfeeding patients
• children.
Administration
• Mix oral solution with water, juice, carbonated beverage, or semisolid food (such as applesauce or pudding) just before administration.
• Remove orally disintegrating tablets with dry hands immediately before administering. After removing, place tablet on patient's tongue, where it will dissolve in approximately 1 minute. Tell patient to swallow saliva.
• Give I.M. or direct I.V. without further dilution.
• Administer low doses (10 mg or less) by direct I.V. injection slowly over 2 minutes. (Rapid injection may cause intense anxiety and restlessness followed by drowsiness.)
• For I.V. infusion, dilute with 50 ml of 5% dextrose in 0.9% sodium chloride solution, 5% dextrose in 0.45% sodium chloride solution, or lactated Ringer's solution. Infuse over at least 15 minutes.
Adverse reactions
CNS: drowsiness, restlessness, anxiety, depression, irritability, fatigue, lassitude, insomnia, tardive dyskinesia, parkinsonian-like reactions, extrapyramidal reactions, akathisia, dystonia
CV: hypertension, hypotension, arrhythmias, neuroleptic malignant syndrome
GI: nausea, constipation, diarrhea, dry mouth
GU: gynecomastia
Interactions
Drug-drug. Anticholinergics, opioids: antagonism of metoclopramide's GI motility effect
Antidepressants, antihistamines, other CNS depressants (such as opioids, sedative-hypnotics): additive CNS depression
Cimetidine, digoxin: decreased blood levels of these drugs
General anesthestics: exaggerated hypotension
Haloperidol, phenothiazines: increased risk of extrapyramidal reactions
Levodopa: decreased metoclopramide efficacy
MAO inhibitors: increased catecholamine release
Drug-diagnostic tests. Aldosterone, prolactin: increased levels
Drug-behaviors. Alcohol use: increased blood alcohol level, increased CNS depression
Patient monitoring
• Monitor blood pressure during I.V. administration.
• Stay alert for depression and other adverse CNS effects.
☞ Watch for extrapyramidal reactions, which usually occur within first 24 to 48 hours of therapy. To reverse these symptoms, give diphenhydramine 50 mg I.M. or benztropine 1 to 2 mg I.M., as prescribed.
• Check for development of parkinsonian-like symptoms, which may occur within first 6 months of therapy and usually subside within 2 to 3 months after withdrawal.
☞ With long-term use, assess patient for tardive dyskinesia and discontinue drug if signs or symptoms of tardive dyskinesia develop. Avoid treatment for longer than 12 weeks in all but rare cases in which therapeutic benefit outweighs risk of developing tardive dyskinesia.
☞ Monitor patient closely for signs and symptoms of neuroleptic malignant syndrome (hyperthermia, muscle rigidity, altered consciousness, and evidence of autonomic instability [irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac arrhythmias]); immediately discontinue drug if these symptoms occur.
• In diabetic patient, stay alert for gastric stasis. Insulin dosage may need to be adjusted.
Patient teaching
• Tell patient to take 30 minutes before meals.
• Tell patient taking orally disintegrating tablets to remove tablet with dry hands immediately before use. Instruct patient to place tablet on tongue, where it will dissolve in approximately 1 minute, and then swallow saliva.
☞ Instruct patient to report involuntary movements of face, eyes, or limbs; muscle rigidity; altered consciousness; irregular pulse or blood pressure; rapid or irregular heartbeats; or excessive sweating.
• Caution patient to avoid driving and other hazardous activities until drug's effects are known.
• As appropriate, review all other significant and life-threatening adverse reactions and interactions, especially those related to the drugs, tests, and behaviors mentioned above.
Latest Searches:
Voraxaze - Voranil - Voorhoeve - voodoo - VOO - Vontrol - von - vomitus - vomiturition - vomitory - vomitoria - vomito - vomitive - vomiting - vomit - vomica - vomerovaginalis - vomerovaginal - vomerorostralis - vomerorostral -
- Service manuals - MBI Corp