Medical term:
PhosLo
calcium
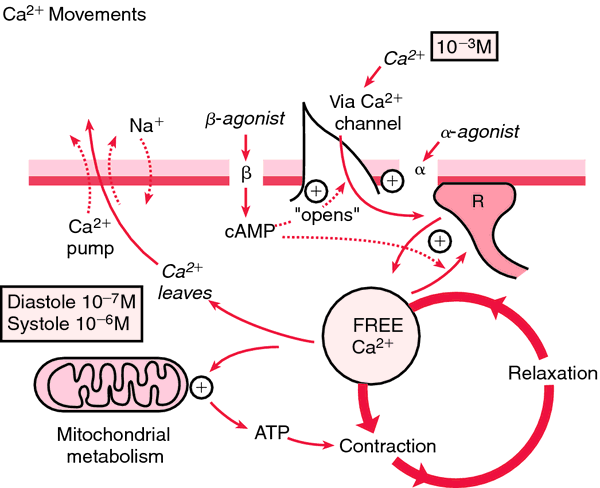
(Ca) [kal´se-um]Within the body fluids calcium exists in three forms. Protein-bound calcium accounts for about 47 per cent of the calcium in plasma; most of it in this form is bound to albumin. Another 47 per cent of plasma calcium is ionized. About 6 per cent is complexed with phosphate, citrate, and other anions.
Ionized calcium is physiologically active. One of its most important physiological functions is control of the permeability of cell membranes. Parathyroid hormone, which causes transfer of exchangeable calcium from bone into the blood stream, maintains calcium homeostasis by preventing either calcium deficit or excess.
Hypercalcemia: This is when the level of serum calcium rises above normal; neuromuscular activity begins to diminish. Symptoms include lethargy, muscle weakness (which, as the level of calcium increases, can progress to depressed reflexes and hypotonic muscles), constipation, mental confusion, and coma. The heartbeat also slows, which potentiates the effects of digitalis.
Hypocalcemia: This is a serum level of calcium that is below normal; it is manifested by increased neuromuscular irritability. When there is a deficit of ionized calcium, the nerve cells become more permeable, allowing leakage of sodium and potassium from the cells. This produces excitation of the nerve fibers and triggers uncontrollable activity of the skeletal muscles. Hence, as the calcium level continues to drop, the patient begins to experience muscle twitching and cramping, grimacing, and carpopedal spasm, which can quickly progress to tetany, laryngospasm, convulsions, cardiac arrhythmias, and eventually to respiratory and cardiac arrest. Relatively early signs of hypocalcemia are a positive trousseau's sign and a positive chvostek's sign.
Dietary sources of calcium include dairy products (such as milk and cheese), soybeans, fortified orange juice, dark green leafy vegetables (such as mustard greens and broccoli), sardines, clams, and oysters. The recommended dietary allowance of calcium for children aged 4 to 8 is 800 mg, and that for women aged 50 to 70 is 1200 mg. (See tables in the Appendices for recommended dietary allowances across the life span.) It is difficult to meet these requirements without including milk or milk products in the daily diet. The most familiar calcium deficiency disease is rickets, in which the bones and teeth soften. However, it is believed that a large number of people suffer from subclinical calcium deficiency because of poor eating habits. Since calcium is essential to the formation and maintenance of strong bones, an adequate intake is important in the prevention of osteoporosis.
calcium acetate
calcium carbonate
calcium chloride
calcium citrate
calcium gluconate
calcium lactate
tricalcium phosphate
Pharmacologic class: Mineral
Therapeutic class: Dietary supplement, electrolyte replacement agent
Pregnancy risk category C (calcium acetate, chloride, glubionate, gluceptate, phosphate), NR (calcium carbonate, citrate, gluconate, lactate)
Action
Increases serum calcium level through direct effects on bone, kidney, and GI tract. Decreases osteoclastic osteolysis by reducing mineral release and collagen breakdown in bone.
Availability
Calcium acetate-
Gelcaps: 667 mg
Tablets: 667 mg
Calcium carbonate-
Capsules: 1,250 mg
Lozenges: 600 mg
Oral suspension: 1,250 mg
Powder: 6.5 g
Tablets: 650 mg, 1,250 mg, 1,500 mg
Tablets (chewable): 750 mg, 1,000 mg, 1,250 mg
Tablets (gum): 300 mg, 450 mg, 500 mg
Calcium chloride-
Injection: 10% solution
Calcium citrate-
Tablets: 950 mg
Calcium gluceptate-
Injection: 22% solution
Calcium gluconate-
Injection: 10% solution
Tablets: 500 mg, 650 mg, 975 mg
Calcium lactate-
Tablets: 325 mg, 650 mg
Tricalcium phosphate-
Tablets: 600 mg
Indications and dosages
➣ Hypocalcemic emergency
Adults: 7 to 14 mEq I.V. of 10% calcium gluconate solution, 2% to 10% calcium chloride solution, or 22% calcium gluceptate solution
Children: 1 to 7 mEq calcium gluconate I.V.
Infants: Up to 1 mEq calcium gluconate I.V.
➣ Hypocalcemic tetany
Adults: 4.5 to 16 mEq calcium gluconate I.V., repeated as indicated until tetany is controlled
Children: 0.5 to 0.7 mEq/kg calcium gluconate I.V. three to four times daily as indicated until tetany is controlled
Neonates: 2.4 mEq/kg calcium gluconate I.V. daily in divided doses
➣ Cardiac arrest
Adults: 0.027 to 0.054 mEq/kg calcium chloride I.V., 4.5 to 6.3 mEq calcium gluceptate I.V., or 2.3 to 3.7 mEq calcium gluconate I.V.
Children: 0.27 mEq/kg calcium chloride I.V., repeated in 10 minutes if needed. Check calcium level before giving additional doses.
➣ Magnesium intoxication
Adults: Initially, 7 mEq I.V.; subsequent dosages based on patient response
➣ Exchange transfusions
Adults: 1.35 mEq calcium gluconate I.V. with each 100 ml of citrated blood
➣ Hyperphosphatemia in patients with end-stage renal disease
Adults: Two tablets P.O. daily, given in divided doses t.i.d. with meals. May increase gradually to bring serum phosphate level below 6 mg/dl, provided hypercalcemia doesn't develop.
➣ Dietary supplement
Adults: 500 mg to 2 g P.O. daily
Off-label uses
• Osteoporosis
Contraindications
• Hypersensitivity to drug
• Ventricular fibrillation
• Hypercalcemia and hypophosphatemia
• Cancer
• Renal calculi
• Pregnancy or breastfeeding
Precautions
Use cautiously in:
• renal insufficiency, pernicious anemia, heart disease, sarcoidosis, hyperparathyroidism, hypoparathyroidism
• history of renal calculi
• children.
Administration
☞ When infusing I.V., don't exceed a rate of 200 mg/minute.
• Keep patient supine for 15 minutes after I.V. administration to prevent orthostatic hypotension.
• Administer P.O. doses 1 to 1½ hours after meals.
• Know that I.M. or subcutaneous administration is never recommended.
• Be aware that I.V. route is preferred in children.
• Be alert for extravasation, which causes tissue necrosis.
Adverse reactions
CNS: headache, weakness, dizziness, syncope, paresthesia
CV: mild blood pressure decrease, bradycardia, arrhythmias, cardiac arrest (with rapid I.V. injection)
GI: nausea, vomiting, diarrhea, constipation, epigastric pain or discomfort
GU: urinary frequency, renal calculi
Metabolic: hypercalcemia
Musculoskeletal: joint pain, back pain
Respiratory: dyspnea
Skin: rash
Other: altered or chalky taste, excessive thirst, allergic reactions (including facial flushing, swelling, tingling, tenderness in hands, and anaphylaxis)
Interactions
Drug-drug. Atenolol, fluoroquinolones, tetracycline: decreased bioavailability of these drugs
Calcium channel blockers: decreased calcium effects
Cardiac glycosides: increased risk of cardiac glycoside toxicity
Iron salts: decreased iron absorption
Sodium polystyrene sulfonate: metabolic alkalosis
Verapamil: reversal of verapamil effects
Drug-diagnostic tests. Calcium: increased level
Drug-food. Foods containing oxalic acid (such as spinach), phytic acid (such as whole grain cereal), or phosphorus (such as dairy products): interference with calcium absorption
Patient monitoring
• Monitor calcium levels frequently, especially in elderly patients.
Patient teaching
• Instruct patient to consume plenty of milk and dairy products during therapy.
• Refer patient to dietitian for help in meal planning and preparation.
• As appropriate, review all other significant and life-threatening adverse reactions and interactions, especially those related to the drugs, tests, and foods mentioned above.
calcium acetate
(kal-see-um ass -e-tate) ,Eliphos
(trade name),PhosLo
(trade name),Phoslyra
(trade name)Classification
Therapeutic: mineral electrolyte replacements supplementsIndications
Action
Therapeutic effects
Pharmacokinetics
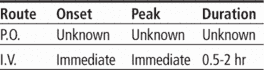
Time/action profile (effects on serum calcium)
| ROUTE | ONSET | PEAK | DURATION |
|---|---|---|---|
| PO | unknown | unknown | unknown |
Contraindications/Precautions
Adverse Reactions/Side Effects
Central nervous system
- headache
- tingling
Cardiovascular
- arrhythmias (most frequent)
- bradycardia
Fluid and Electrolyte
- hypercalcemia (most frequent)
Gastrointestinal
- constipation (most frequent)
- diarrhea (oral solution only)
- nausea
- vomiting
Genitourinary
- calculi
- hypercalciuria
Interactions
Drug-Drug interaction
Hypercalcemia ↑ risk of digoxin toxicity.Chronic use with antacids in renal insufficiency may lead to milk-alkali syndrome.Calcium supplements, including calcium-containing antacids may ↑ risk of hypercalcemia; avoid concurrent use.May ↓ absorption of orally administered tetracyclines, fluoroquinolones, phenytoin, and iron salts ; take 1 hr before or 3 hr after calcium acetate.Excessive amounts may ↓ effects of calcium channel blockers.↓ absorption of etidronate and risedronate (do not take within 2 hr of calcium acetate).May ↓ effectiveness of atenolol.Concurrent use with diuretics (thiazide) may result in hypercalcemia.May ↓ ability of sodium polystyrene sulfonate to ↓ serum potassium.Cereals, spinach, or rhubarb may ↓ absorption of calcium supplements.Route/Dosage
1 gram of calcium acetate contains 250 mg elemental calcium (12.7 mEq calcium). Doses are expressed in mg calcium acetateAvailability (generic available)
Nursing implications
Nursing assessment
- .
- Monitor patient on digitalis glycosides for signs of toxicity.
- Lab Test Considerations: Monitor serum calcium twice weekly during adjustment phase. If serum calcium level is >12 mg/dL, discontinue therapy and start hemodialysis as needed; lower dose or temporarily stop therapy for calcium level between 10.5 to 11.9 mg/dL.
- Monitor serum phosphate levels to determine efficacy.
Potential Nursing Diagnoses
Imbalanced nutrition: less than body requirements (Indications)Implementation
- Oral: Administer on an empty stomach before meals to optimize effectiveness in patients with hyperphosphatemia.
Patient/Family Teaching
- Instruct patients on a regular schedule to take missed doses as soon as possible, then go back to regular schedule.
- Advise patient to notify health care professional promptly if signs and symptoms of hypercalcemia (constipation, anorexia, nausea, vomiting, confusion, stupor) occur.
- Advise patient to avoid taking calcium-containing supplements, including calcium-based antacids during therapy.
Evaluation/Desired Outcomes
- Control of hyperphosphatemia in patients with renal failure.
calcium acetate
An agent which is used to lower serum phosphate levels in patients with hyperphosphataemia; in patients with end-stage renal disease; neutralise fluoride in fluoridated water; and as a food additive, in which it acts as a stabiliser, buffer and sequestrant.Latest Searches:
Voraxaze - Voranil - Voorhoeve - voodoo - VOO - Vontrol - von - vomitus - vomiturition - vomitory - vomitoria - vomito - vomitive - vomiting - vomit - vomica - vomerovaginalis - vomerovaginal - vomerorostralis - vomerorostral -
- Service manuals - MBI Corp