Medical term:
Prelone
prednisolone
prednisolone acetate
prednisolone sodium phosphate
Pharmacologic class: Corticosteroid (intermediate-acting)
Therapeutic class: Anti-inflammatory, immunosuppressant
Pregnancy risk category C
Action
Exerts potent anti-inflammatory (glucocorticoid) and weak sodium-retaining (mineralocorticoid) activity. Glucocorticoid activity causes profound and varied metabolic effects.
Availability
Oral solution: 5 mg/ml
Suspension for injection (acetate): 25 mg/ml, 40 mg/ml, 50 mg/ml
Suspension (ophthalmic): 0.12%, 0.125%, 1%
Syrup: 5 mg/5 ml, 15 mg/5 ml
Tablets: 5 mg
Tablets (orally disintegrating, sodium phosphate): 10 mg, 15 mg, 30 mg
Indications and dosages
➣ Severe inflammation; immunosuppression
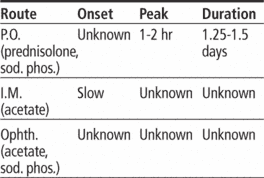
Adults: Dosage individualized based on diagnosis, severity of condition, and response. Usual dosage ranges from 5 to 60 mg P.O. (prednisolone) daily in two to four divided doses, or 4 to 60 mg I.M. (acetate) daily in divided doses, or 5 to 50 mg P.O. (sodium phosphate) daily in divided doses.
➣ Acute exacerbation of multiple sclerosis
Adults: 200 mg P.O. daily for 1 week, followed by 80 mg every other day for 1 month
➣ Refractory bronchial asthma
Children: 1 to 2 mg/kg/day (sodium phosphate) as a single dose or in divided doses; may continue for 3 to 10 days or until symptoms resolve or patient achieves peak expiratory flow rate of 80% of personal best
➣ Nephrotic syndrome in children
Children: 60 mg/m2 P.O. (sodium phosphate solution) daily in three divided doses for 4 weeks, then 4 weeks of alternate-day therapy at single doses of 40 mg/m2
➣ Various allergic conditions and dermatologic, endocrine, GI, hematologic, neoplastic, nervous system, ophthalmic, respiratory, and rheumatic disorders
Adults: Variable and individualized depending on condition being treated and patient response. Initially, 10 to 60 mg (ODT) P.O. daily.
Children: Variable and individualized depending on condition being treated. Initial dosage range is 0.14 to 2 mg/kg/day P.O. in three or four divided doses.
➣ Steroid-responsive inflammatory eye conditions
Adults: In severe cases, initially one to two drops (acetate or sodium phosphate) instilled into conjunctival sac q hour during day and q 2 hours at night. In mild or moderate inflammation or in severe cases that respond favorably, one to two drops q 3 to 12 hours.
Contraindications
• Hypersensitivity to drug, other corticosteroids, alcohol, bisulfite, or tartrazine (with some products)
• Systemic fungal infections
• Active untreated infections (except in selected patients with meningitis)
• Acute superficial herpes simplex, keratitis, fungal or viral eye diseases, tuberculosis of eye, or after uncomplicated removal of superficial corneal foreign body (ophthalmic use)
• Idiopathic thrombocytopenic purpura (with I.M. use)
• Live-virus vaccines (with immunosuppressive prednisolone dosages)
Precautions
Use cautiously in:
• diabetes mellitus, glaucoma, renal or hepatic disease, hypothyroidism, cirrhosis, diverticulitis, nonspecific ulcerative colitis, recent intestinal anastomoses, inflammatory bowel disease, thromboembolic disorders, seizures, myasthenia gravis, heart failure, hypertension, osteoporosis, ocular herpes simplex, immunosuppression, emotional instability
• pregnant or breastfeeding patients
• children younger than age 6 (younger than age 2 when treated for nephrotic syndrome; younger than age 1 month when treated for aggressive lymphomas and leukemias with ODT form).
Administration
☞ Be aware that prednisolone has many different formulations that may be given by various routes: P.O., I.M., or ophthalmic. Before administering, make sure formulation can be given by prescribed route.
• Don't break ODT tablets.
• Place ODT tablet on tongue and either swallow tablet whole or allow it to dissolve in mouth with or without water.
• Inject I.M. form deep into gluteal muscle. Rotate injection sites.
• Avoid subcutaneous injection.
☞ In systemic therapy, don't discontinue drug abruptly, even if inhaled steroid is added.
• Know that additional corticosteroids are needed during stress or trauma.
Adverse reactions
CNS: headache, nervousness, depression, euphoria, personality changes, psychosis, vertigo, paresthesia, insomnia, restlessness, seizures, meningitis, increased intracranial pressure, hypertrophic cardiomyopathy in premature infants
CV: hypotension, hypertension, vasculitis, thrombophlebitis, thrombo-embolism, fat embolism, arrhythmias, heart failure, shock
EENT: cataracts, glaucoma, visual disturbances, exacerbation of ocular infection, secondary ocular infections, globe perforation at corneal or scleral thinning site, transient stinging or burning of eyes, dry eyes, corneal ulcers, mydriasis (all with ophthalmic use); posterior subcapsular cataracts (especially in children), glaucoma, nasal irritation and congestion, rebound congestion, sneezing, epistaxis, nasopharyngeal and oropharyngeal fungal infections, perforated nasal septum, anosmia, dysphonia, hoarseness, throat irritation (with long-term use)
GI: nausea, vomiting, abdominal distention, rectal bleeding, dry mouth, esophageal candidiasis, esophageal ulcer, pancreatitis, peptic ulcer
GU: amenorrhea, irregular menses
Hematologic: purpura
Metabolic: sodium and fluid retention, hypokalemia, hypocalcemia, hyper-glycemia, decreased carbohydrate tolerance, growth retardation (in children), diabetes mellitus, cushingoid effects (with long-term use), hypothal-amic-pituitary-adrenal suppression (with systemic use longer than 5 days), adrenal suppression (with high-dose, long-term use)
Musculoskeletal: muscle weakness or atrophy, myalgia, myopathy, osteoporosis, aseptic joint necrosis, spontaneous fractures (with long-term use), osteonecrosis, tendon rupture
Respiratory: cough, wheezing, bron-chospasm
Skin: urticaria, rash, pruritus, contact dermatitis, acne, striae, poor wound healing, thin fragile skin, bruising, hir-sutism, petechiae, subcutaneous fat atrophy, urticaria, angioedema
Other: aggravation or masking of infections, increased or decreased appetite, weight gain (with long-term use), facial edema and erythema, edema, hypersensitivity reaction
Interactions
Drug-drug. Amphotericin B, mezlocillin, piperacillin, thiazide and loop diuretics, ticarcillin: additive hypokalemia Anticholinesterase drugs: decreased anticholinesterase effect (when prednisolone is used for myasthenia gravis)
Aspirin, other nonsteroidal anti-inflammatory drugs: increased risk of GI discomfort and bleeding
Cardiac glycosides: increased risk of digitalis toxicity due to hypokalemia
Cyclosporine: therapeutic benefits in organ transplant recipients, but with increased risk of toxicity
Erythromycin, indinavir, itraconazole, ketoconazole, ritonavir, saquinavir: increased prednisolone blood level and effects
Hormonal contraceptives: impaired metabolism and increased effects of prednisolone
Isoniazid: decreased isoniazid blood level
Live-virus vaccines: decreased antibody response to vaccine, increased risk of adverse effects
Oral anticoagulants: reduced anticoagulant requirement, opposition to anticoagulant action
Phenobarbital, phenytoin, rifampin: decreased prednisolone efficacy Salicylates: reduced salicylate blood level
Somatrem: inhibition of somatrem's growth-promoting effects
Theophylline: altered pharmacologic effects of either drug
Skin tests: suppressed results
Drug-diagnostic tests. Calcium, potassium, thyroid 131I uptake, thyroxine, tri-iodothyronine: decreased levels
Cholesterol, glucose: increased levels Nitroblue tetrazolium test for bacterial infection: false-negative result
Skin tests: suppressed results
Drug-herbs. Alfalfa: activation of quiescent systemic lupus erythematosus
Echinacea: increased immune-stimulating effects
Ephedra (ma huang): decreased drug blood level
Ginseng: potentiation of immunomodulating effect
Licorice: prolonged drug activity
Drug-behaviors. Alcohol use: increased risk of gastric irritation and GI ulcers
Patient monitoring
• Monitor weight, blood pressure, and electrolyte levels.
• Watch for cushingoid effects (moon face, central obesity, buffalo hump, hair thinning, high blood pressure, frequent infections).
☞ Assess patient for depression and psychosis.
• Monitor blood glucose level carefully in diabetic patient.
• Evaluate for signs and symptoms of infection, which drug may mask or exacerbate.
☞ Monitor for signs and symptoms of early adrenal insufficiency (fatigue, weakness, joint pain, fever, anorexia, shortness of breath, dizziness, syncope).
• Assess musculoskeletal status for joint, tendon, and muscle pain.
Patient teaching
• Tell patient to take oral dose with food or milk to reduce GI upset.
• Instruct patient to remove ODT tablet from blister just before taking.
• Instruct patient to place ODT tablet on tongue and either swallow tablet whole or allow it to dissolve in mouth with or without water. Caution patient not to cut, split, or break tablet.
☞ Teach patient to recognize and immediately report cushingoid effects and signs and symptoms of early adrenal insufficiency.
☞ Advise patient and significant other to immediately report depression or psychosis.
• Explain that drug increases risk of infection. Instruct patient to contact prescriber at first sign of infection.
☞ Caution patient not to suddenly stop drug (including ophthalmic forms). Instruct him to discuss any changes in therapy with prescriber.
☞ Tell patient to immediately report bleeding or joint, muscle, tendon, or abdominal pain.
• Inform patient that he may need higher dosage during periods of stress. Encourage him to wear or carry medical identification stating this.
• Tell patient to avoid vaccinations during therapy. Mention that others in household shouldn't receive oral polio vaccine because they could pass poliovirus to him.
• Caution patient not to take over-the-counter drugs or herbs during therapy.
• Teach patient how to use eye drops. Caution him not to touch dropper tip to eye or any other surface.
• As appropriate, review all other significant and life-threatening adverse reactions and interactions, especially those related to the drugs, tests, herbs, and behaviors mentioned above.
prednisoLONE
(pred-niss-oh-lone) ,Flo-Pred
(trade name),Orapred
(trade name),Orapred ODT
(trade name),Pediapred
(trade name),Prelone
(trade name)Classification
Therapeutic: anti inflammatories steroidalIndications
- Inflammatory,
- Allergic,
- Hematologic,
- Neoplastic,
- Autoimmune disorders.
Action
Therapeutic effects
Pharmacokinetics
Time/action profile (anti-inflammatory activity)
| ROUTE | ONSET | PEAK | DURATION |
|---|---|---|---|
| PO | unknown | 1–2 hr | 1.25–1.5 days |
Contraindications/Precautions
Adverse Reactions/Side Effects
Adverse reactions/side effects are much more common with high-dose/long-term therapyCentral nervous system
- depression (most frequent)
- euphoria (most frequent)
- headache
- ↑ intracranial pressure (children only)
- personality changes
- psychoses
- restlessness
Ear, Eye, Nose, Throat
- cataracts
- ↑ intraocular pressure
Cardiovascular
- hypertension (most frequent)
Gastrointestinal
- peptic ulceration (life-threatening)
- anorexia (most frequent)
- nausea (most frequent)
- vomiting
Dermatologic
- acne (most frequent)
- ↓ wound healing (most frequent)
- ecchymoses (most frequent)
- fragility (most frequent)
- hirsutism (most frequent)
- petechiae (most frequent)
Endocrinologic
- adrenal suppression (most frequent)
- hyperglycemia
Fluid and Electrolyte
- fluid retention (long-term high doses)
- hypokalemia
- hypokalemic alkalosis
Hematologic
- thromboembolism (life-threatening)
- thrombophlebitis
Metabolic
- weight gain
- weight loss
Musculoskeletal
- muscle wasting (most frequent)
- osteoporosis (most frequent)
- avascular necrosis of joints
- muscle pain
Miscellaneous
- cushingoid appearance (moon face, buffalo hump) (most frequent)
- ↑ susceptibility to infection
Interactions
Drug-Drug interaction
Additive hypokalemia with thiazide and loop diuretics, amphotericin B, piperacillin, or ticarcillin.Hypokalemia may ↑ risk of digoxin toxicity.May ↑ requirement for insulin or oral hypoglycemic agents.Phenytoin, phenobarbital, and rifampin stimulate metabolism; may ↓ effectiveness.Oral contraceptives may ↓ metabolism.↑ risk of adverse GI effects withNSAIDs (including aspirin ).At chronic doses that suppress adrenal function, may ↓ antibody response to and ↑ risk of adverse reactions from live-virus vaccines.May ↑ risk of tendon rupture from fluoroquinolones.Route/Dosage
Availability (generic available)
Nursing implications
Nursing assessment
- Indicated for many conditions. Assess involved systems prior to and periodically during therapy.
- Assess patient for signs of adrenal insufficiency (hypotension, weight loss, weakness, nausea, vomiting, anorexia, lethargy, confusion, restlessness) prior to and periodically during therapy.
- Monitor intake and output ratios and daily weights. Observe patient for peripheral edema, steady weight gain, rales/crackles, or dyspnea. Notify health care professional should these occur.
- Pediatric: Children should have periodic evaluations of growth.
- Lab Test Considerations: Monitor serum electrolytes and glucose. May cause hyperglycemia, especially in persons with diabetes. May cause hypokalemia. Patients on prolonged therapy should routinely have hematologic values, serum electrolytes, and serum and urine glucose evaluated. May ↓ WBC counts. May ↓ serum potassium and calcium and increase serum sodium concentrations.
- Guaiac test stools. Promptly report presence of guaiac-positive stools.
- May ↑ serum cholesterol and lipid values. May ↓ uptake of thyroid 123I or 131I.
- Suppresses reactions to allergy skin tests.
- Periodic adrenal function tests may be ordered to assess degree of hypothalamic-pituitary-adrenal axis suppression in systemic and chronic topical therapy.
Potential Nursing Diagnoses
Risk for infection (Side Effects)Disturbed body image (Side Effects)
Deficient knowledge, related to medication regimen (Patient/Family Teaching)
Implementation
- If dose is ordered daily or every other day, administer in the morning to coincide with the body's normal secretion of cortisol.
- Periods of stress, such as surgery, may require supplemental systemic corticosteroids.
- Oral: Administer with meals or milk to minimize GI irritation.
- Tablets may be crushed and administered with food or fluids for patients with difficulty swallowing.
- Orally disintegrating tablets: Remove tablet from blister just prior to dosing. Peel blister pack open, and place orally disintegrating tablet on tongue. Tablets may be swallowed whole or allowed to dissolve in mouth, with or without water. Do not cut, split, or break.
- Use calibrated measuring device to ensure accurate dose of liquid forms.
Patient/Family Teaching
- Instruct patient on correct technique of medication administration. Advise patient to take medication as directed. Take missed doses as soon as remembered unless almost time for next dose. Do not double doses. Stopping the medication suddenly may result in adrenal insufficiency (anorexia, nausea, weakness, fatigue, dyspnea, hypotension, hypoglycemia). If these signs appear, notify health care professional immediately. This can be life-threatening.
- Glucocorticoids cause immunosuppression and may mask symptoms of infection. Instruct patient to avoid people with known contagious illnesses and to report possible infections immediately.
- Prelone syrup should not be refrigerated, Pediapred solution may be refrigerated, Orapred solution should be refrigerated.
- Caution patient to avoid vaccinations without first consulting health care professional.
- Review side effects with patient. Instruct patient to inform health care professional promptly if severe abdominal pain or tarry stools occur Patient should also report unusual swelling, weight gain, tiredness, bone pain, bruising, nonhealing sores, visual disturbances, or behavior changes.
- Advise patient to notify health care professional of medication regimen prior to treatment or surgery.
- Discuss possible effects on body image. Explore coping mechanisms.
- Instruct patient to inform health care professional if symptoms of underlying disease return or worsen.
- Advise patient to carry identification describing disease process and medication regimen in the event of emergency in which patient cannot relate medical history.
- Explain need for continued medical follow-up to assess effectiveness and possible side effects of medication. Periodic lab tests and eye exams may be needed.
- Long-term Therapy: Encourage patient to eat a diet high in protein, calcium, and potassium, and low in sodium and carbohydrates (see ). Alcohol should be avoided during therapy.
Evaluation/Desired Outcomes
- Decrease in presenting symptoms with minimal systemic side effects.
- Suppression of the inflammatory and immune responses in autoimmune disorders, allergic reactions, and neoplasms.
- Management of symptoms in adrenal insufficiency.
Prelone
(prē′lōn′)Prelone®
Prednisone, see there.Latest Searches:
Voraxaze - Voranil - Voorhoeve - voodoo - VOO - Vontrol - von - vomitus - vomiturition - vomitory - vomitoria - vomito - vomitive - vomiting - vomit - vomica - vomerovaginalis - vomerovaginal - vomerorostralis - vomerorostral -
- Service manuals - MBI Corp