amino
[ah-me´no, am´ĭ-no] the monovalent radical NH2, when not united with an acid radical.
amino acid any of a class of organic compounds containing the amino (NH
2) and the carboxyl (COOH) groups, occurring naturally in plant and animal tissues and forming the chief constituents of
protein. Twenty amino acids are necessary for protein synthesis. Eleven (the
nonessential amino acids) can be synthesized by the human body and thus are not specifically required in the diet:
alanine,
arginine,
asparagine,
aspartic acid,
cysteine,
glutamic acid,
glutamine,
glycine,
proline,
serine, and
tyrosine. Nine (the
essential amino acids) cannot be synthesized by humans and thus are required in the diet:
histidine,
isoleucine,
leucine,
lysine,
methionine,
phenylalanine,
threonine,
tryptophan, and
valine.
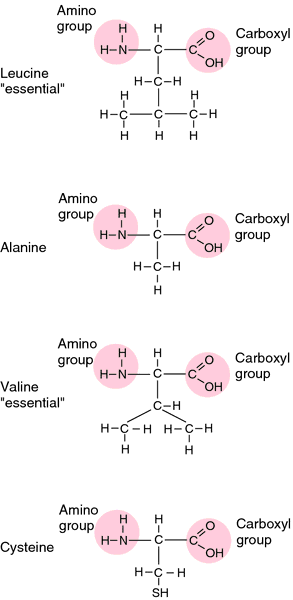
Structural formulas for some representative amino acids. From Applegate, 2000.
Protein foods that provide the essential amino acids are known as
complete proteins; these include proteins from animal sources, such as meat, eggs, fish, and milk. Proteins that cannot supply the body with all the essential amino acids are known as
incomplete proteins; these are the vegetable proteins most abundantly found in
legumes (peas and beans), as well as certain grains. Because different incomplete proteins lack different amino acids, specific combinations can provide all of the essential amino acids.
In certain inherited or acquired disorders of metabolism, specific amino acids accumulate in the blood
(aminoacidemia) or are excreted in excess in the urine
(aminoaciduria). Urinary amino acid levels are increased in liver disease, muscular dystrophies, phenylketonuria (PKU), lead poisoning, and folic acid deficiency.
Miller-Keane Encyclopedia and Dictionary of Medicine, Nursing, and Allied Health, Seventh Edition. © 2003 by Saunders, an imprint of Elsevier, Inc. All rights reserved.
a·mi·no ac·id (AA, aa),
(ă-mē'nō as'id), An organic acid in which one of the hydrogen atoms on a carbon atom has been replaced by NH2. Usually refers to an aminocarboxylic acid. However, taurine is also an amino acid.
See also: α-amino acid.
Farlex Partner Medical Dictionary © Farlex 2012
amino acid
n. Any of various compounds containing an amino group (NH2), a carboxylic acid group (COOH), and a distinctive side chain, especially any of the 20 amino acids that link together to form proteins. Some amino acids (called nonessential) can be synthesized in the human body, while others (called essential) must be obtained through the diet.
The American Heritage® Medical Dictionary Copyright © 2007, 2004 by Houghton Mifflin Company. Published by Houghton Mifflin Company. All rights reserved.
ami·no ac·id
(AA, aa) (ă-mē'nō as'id) An organic acid in which one of the hydrogen atoms on a carbon atom has been replaced by NH2. Usually refers to an aminocarboxylic acid. However, taurine is also an amino acid.
See also: alpha (α)-amino acid
Medical Dictionary for the Health Professions and Nursing © Farlex 2012
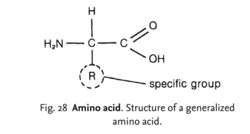
Fig. 28 Amino acid . Structure of a generalized amino acid.

Fig. 27 Amino acid . The 20 amino acids commonly found in protein, with the three-letter and, in brackets, one-letter abbreviation for each.
amino acid
a building block of protein, containing a carboxyl group (COOH) and an amino group (NH2), both attached to the same carbon atom. Over 80 amino acids are known to occur naturally, with 20 found commonly in proteins (see Fig. 27 ), each with a different side chain, called an ‘R’ group (see Fig. 28 ). Each of these common amino acids is described under its own heading. Many amino acids can be synthesized in the body from other amino acids by a process called TRANSAMINATION, although most organisms have a number of ESSENTIAL AMINO ACIDS that must be taken in with the diet. Each amino acid is coded by at least one triplet of DNA bases (see GENETIC CODE), and the string of amino acids making up a protein is joined by PEPTIDE BONDS to form a POLYPEPTIDE CHAIN. The sequence of amino acids is the PRIMARY STRUCTURE. Amino acids are soluble in water but vary considerably in their solubility. When in solution they are ionized (see ZWITTERION) and generally are electrically neutral with a pH known as the ISOELECTRIC POINT. They are amphoteric, i.e. acting as acids or bases if the pH is shifted.
Collins Dictionary of Biology, 3rd ed. © W. G. Hale, V. A. Saunders, J. P. Margham 2005
Amino acid
Amino acids are small molecules that are used as building blocks for all proteins. Some amino acids are also used in the body for the manufacture of hormones. There are about 20 nutritionally important amino acids, including glutamic acid, glycine, methionine, lysine, tryptophan, serine, and glycine.
Mentioned in: Amino Acid Disorders Screening, Cystinuria, Homocysteine, Phenylketonuria, Sickle Cell Disease, Stress Reduction, Vitamin B 6 Deficiency
Gale Encyclopedia of Medicine. Copyright 2008 The Gale Group, Inc. All rights reserved.
ami·no ac·id
(aa) (ă-mē'nō as'id) An organic acid in which one of the hydrogen atoms on a carbon atom has been replaced by NH2. Usually refers to an aminocarboxylic acid. However, taurine is also an amino acid.
See also: alpha (α)-amino acid
Medical Dictionary for the Dental Professions © Farlex 2012
Patient discussion about Amino acid
Q. what are Amino Acids and what are their for? how do i need to do to keep it "going "?
A. Amino acids are the basic structural building units of proteins. They form short polymer chains called peptides or longer chains called either polypeptides or proteins. The process of such formation from an mRNA template is known as translation, which is part of protein biosynthesis. Twenty amino acids are encoded by the standard genetic code and are called proteinogenic or standard amino acids. Other amino acids contained in proteins are usually formed by post-translational modification, which is modification after translation in protein synthesis. These modifications are often essential for the function or regulation of a protein; for example, the carboxylation of glutamate allows for better binding of calcium cations, and the hydroxylation of proline is critical for maintaining connective tissues and responding to oxygen starvation. For full article: http://en.wikipedia.org/wiki/Amino_acid Hope this helps.
More discussions about Amino acidThis content is provided by iMedix and is subject to iMedix Terms. The Questions and Answers are not endorsed or recommended and are made available by patients, not doctors.


