Medical term:
osmotic
os·mot·ic
(oz-mot'ik),Relating to osmosis.
Synonym(s): osmolar
Farlex Partner Medical Dictionary © Farlex 2012
os·mot·ic
(oz-mot'ik)Relating to osmosis.
Synonym(s): osmolar.
Synonym(s): osmolar.
Medical Dictionary for the Health Professions and Nursing © Farlex 2012
osmosis
(oz-mō′sĭs) [Gr. osmos, impulse, + osis, condition]
OSMOSIS
The passage of solvent through a semipermeable membrane that separates solutions of different concentrations. The solvent, usually water, passes through the membrane from the region of lower concentration of solute to that of a higher concentration of solute, thus tending to equalize the concentrations of the two solutions. The rate of osmosis is dependent primarily upon the difference in osmotic pressures of the solutions on the two sides of a membrane, the permeability of the membrane, and the electric potential across the membrane and the charge upon the walls of the pores in it. See: illustration
reverse osmosis
A form of water treatment that removes infectious particles and dissolved ions more effectively than other water purification techniques. Water so purified can be used in hemodialysis.
osmotic (oz-mot′ik), adjectiveillustrationMedical Dictionary, © 2009 Farlex and Partners
os·mot·ic
(os-mot'ik)Relating to osmosis.
Synonym(s): osmolar.
Synonym(s): osmolar.
Medical Dictionary for the Dental Professions © Farlex 2012
osmosis
[oz-mo´sis, os-mo´sis]the diffusion of pure solvent across a membrane in response to a concentration gradient, usually from a solution of lesser to one of greater solute concentration. adj., adj osmot´ic.
The process of osmosis and the factors that influence it are important clinically in the maintenance of adequate body fluids and in the proper balance between volumes of extracellular and intracellular fluids.
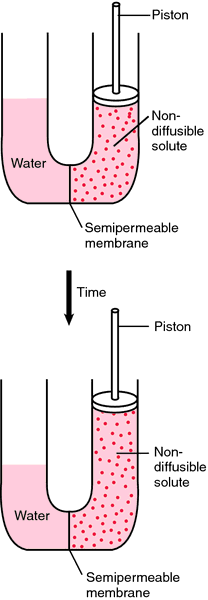
The term osmotic pressure refers to the amount of pressure necessary to stop the flow of water across the membrane. The hydrostatic pressure of the water exerts an opposite effect; that is, it exerts pressure in favor of the flow of water across the membrane. The osmotic pressure of the particles in a solute depends on the relative concentrations of the solutions on either side of the membrane, and on the area of the membrane. The osmotic pressure exerted by the nondiffusible particles in a solution is determined by the numbers of particles in a unit of fluid and not by the mass of the particles.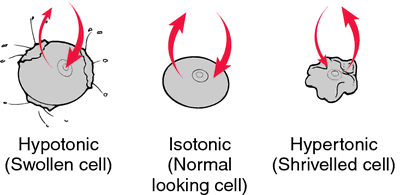
The process of osmosis and the factors that influence it are important clinically in the maintenance of adequate body fluids and in the proper balance between volumes of extracellular and intracellular fluids.
The term osmotic pressure refers to the amount of pressure necessary to stop the flow of water across the membrane. The hydrostatic pressure of the water exerts an opposite effect; that is, it exerts pressure in favor of the flow of water across the membrane. The osmotic pressure of the particles in a solute depends on the relative concentrations of the solutions on either side of the membrane, and on the area of the membrane. The osmotic pressure exerted by the nondiffusible particles in a solution is determined by the numbers of particles in a unit of fluid and not by the mass of the particles.

If the solution surrounding a cell has the same solute concentration as the internal environment of the cell (isotonic), the flow rates in and out of the cell are the same, and the cell remains the same size. If the solute concentration outside the cell is lower (hypotonic), more water will flow into the cell than out, and the cell will swell and perhaps burst. If the solute concentration outside the cell is greater (hypertonic), more water will flow out of the cell than into it, and the cell will shrivel.

Demonstration of osmotic pressure on the two sides of a semipermeable membrane.
Miller-Keane Encyclopedia and Dictionary of Medicine, Nursing, and Allied Health, Seventh Edition. © 2003 by Saunders, an imprint of Elsevier, Inc. All rights reserved.
os·mo·sis
(os-mō'sis),The process by which solvent tends to move through a semipermeable membrane from a solution of lower to a solution of higher osmolal concentration of the solutes to which the membrane is relatively impermeable.
[G. ōsmos, a thrusting, an impulsion]
Farlex Partner Medical Dictionary © Farlex 2012
osmosis
(ŏz-mō′sĭs, ŏs-)n. pl. osmo·ses (-sēz)
1.
a. Diffusion of fluid through a semipermeable membrane from a solution with a low solute concentration to a solution with a higher solute concentration until there is an equal solute concentration on both sides of the membrane.
b. The tendency of fluids to diffuse in such a manner.
2. A gradual, often unconscious process of assimilation or absorption: learned French by osmosis while residing in Paris for 15 years.
os·mot′ic (-mŏt′ĭk) adj.
os·mot′i·cal·ly adv.
The American Heritage® Medical Dictionary Copyright © 2007, 2004 by Houghton Mifflin Company. Published by Houghton Mifflin Company. All rights reserved.
os·mo·sis
(oz-mō'sis)The process by which solvent tends to move through a semipermeable membrane from a solution of lower to a solution of higher osmolal concentration of the solutes to which the membrane is relatively impermeable.
[G. ōsmos, a thrusting, an impulsion]
Medical Dictionary for the Health Professions and Nursing © Farlex 2012
osmosis
(oz-mō′sĭs) [Gr. osmos, impulse, + osis, condition]
OSMOSIS
The passage of solvent through a semipermeable membrane that separates solutions of different concentrations. The solvent, usually water, passes through the membrane from the region of lower concentration of solute to that of a higher concentration of solute, thus tending to equalize the concentrations of the two solutions. The rate of osmosis is dependent primarily upon the difference in osmotic pressures of the solutions on the two sides of a membrane, the permeability of the membrane, and the electric potential across the membrane and the charge upon the walls of the pores in it. See: illustration
reverse osmosis
A form of water treatment that removes infectious particles and dissolved ions more effectively than other water purification techniques. Water so purified can be used in hemodialysis.
osmotic (oz-mot′ik), adjectiveillustrationMedical Dictionary, © 2009 Farlex and Partners
osmosis
The automatic movement of the fluid part of a solution through a membrane, separating two quantities of the solution, in such a direction as to dilute the solution of higher concentration. The membrane is permeable to the liquid but not to the dissolved substance. Such a membrane is said to be semipermeable and membranes of this kind occur widely in the body. Osmosis is an important principle on which much of physiology is based.Collins Dictionary of Medicine © Robert M. Youngson 2004, 2005
osmosis
the movement of a solvent (water in biological systems) through a differentially permeable membrane from a solution with high water concentration and low solute concentration, to one with a low water concentration and high solute concentration.Collins Dictionary of Biology, 3rd ed. © W. G. Hale, V. A. Saunders, J. P. Margham 2005
osmosis
A passive process of movement of water through a semipermeable membrane in response to a concentration gradient, from an area of low solute (e.g. glucose molecules) concentration (i.e. high water concentration) to one of high solute concentration (i.e. low water concentration). The membrane is permeable to water but relatively impermeable to solutes. See osmotic pressure; hypertonic solution.
Millodot: Dictionary of Optometry and Visual Science, 7th edition. © 2009 Butterworth-Heinemann
os·mo·sis
(os-mō'sis)Process by which solvent tends to move through a semipermeable membrane from a solution of lower to a solution of higher osmolal concentration of solutes to which membrane is relatively impermeable.
[G. ōsmos, a thrusting, an impulsion]
Medical Dictionary for the Dental Professions © Farlex 2012
Latest Searches:
veneniferous - venenation - venenata - veneered - veneer - venectomy - venectasia - venation - Venastat - venarum - VenaFlow - venae - venacavography - venacavogram - venacaval - vena - velum - Veltin - Veltap - Velpeau -
- Service manuals - MBI Corp