Medical term:
A1c
hemoglobin
[he´mo-glo″bin]Oxygenated hemoglobin (oxyhemoglobin) is bright red in color; hemoglobin unbound to oxygen (deoxyhemoglobin) is darker. This accounts for the bright red color of arterial blood, in which the hemoglobin is about 97 per cent saturated with oxygen. Venous blood is darker because it is only about 20 to 70 per cent saturated, depending on how much oxygen is being used by the tissues. The affinity of hemoglobin for carbon monoxide is 210 times as strong as its affinity for oxygen. The complex formed (carboxyhemoglobin) cannot transport oxygen. Thus, carbon monoxide poisoning results in hypoxia and asphyxiation.
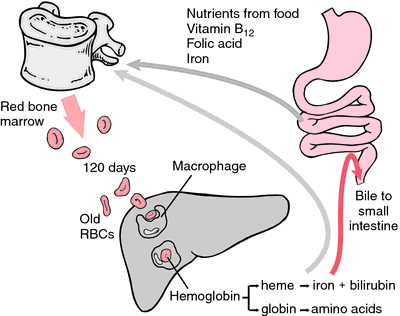
Another form of hemoglobin that cannot transport oxygen is methemoglobin, in which the iron atom is oxidized to the +3 oxidation state. During the 120-day life span of a red blood cell, hemoglobin is slowly oxidized to methemoglobin. At least four different enzyme systems can convert methemoglobin back to hemoglobin. When these are defective or overloaded, methemoglobinemia can result, with high methemoglobin levels causing dyspnea and cyanosis.
A secondary function of hemoglobin is as part of the blood buffer system. The histidine residues in the globin chains act as weak bases to minimize the change in blood pH that occurs as oxygen is absorbed and carbon dioxide released in the lungs and as oxygen is delivered and carbon dioxide taken up from the tissues.
As erythrocytes wear out or are damaged, they are ingested by macrophages of the reticuloendothelial system. The porphyrin ring of heme is converted to the bile pigment bilirubin, which is excreted by the liver. The iron is transported to the bone marrow to be incorporated in the hemoglobin of newly formed erythrocytes.
The hemoglobin concentration of blood varies with the hematocrit. The normal values for the blood hemoglobin concentration are 13.5 to 18.0 g/100 ml in males and 12.0 to 16.0 g/100 ml in females. The normal mean corpuscular hemoglobin concentration, which is the concentration within the red blood cells, is 32 to 36 g/100 ml.
Many abnormal hemoglobins arising from mutations have been discovered. Some have altered oxygen affinity, some are unstable, and in some the iron atom is oxidized, resulting in congenital methemoglobinemia. Some mutations result in a reduced rate of hemoglobin synthesis. All such conditions are known as hemoglobinopathies.
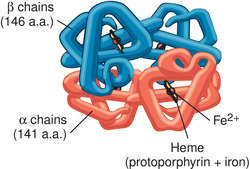
The most common hemoglobinopathy is sickle cell disease, caused by a mutation replacing the sixth amino acid in the β chain, normally glutamic acid, by valine. The variant hemoglobin α2βS2 is known as Hb S. Mutations resulting in reduced synthesis of one of the chains are called thalassemias. They can result from deletion of the gene for a chain or from a mutation in the regulatory gene that controls the synthesis of the chain.
gly·co·syl·at·ed he·mo·glo·bin
gly·co·syl·at·ed he·mo·glo·bin
(glī-kō'si-lāt-ĕd hē'mō-glō-bin)Synonym(s): glycated hemoglobin.
hemoglobin
(he'mo-glo?bin) [ hem- + globin],Hb, Hbg, Hgb
When old RBCs are phagocytized by macrophages in the liver, spleen, and red bone marrow, the iron of hemoglobin is reused immediately to produce new RBCs or is stored in the liver until needed. The globin is converted to amino acids for the synthesis of other proteins. The heme portion is of no further use and is converted to bilirubin.
Hemoglobin combines with carbon monoxide (in carbon monoxide poisoning) to form the stable compound carboxyhemoglobin, which renders hemoglobin unable to bond with oxygen and results in hypoxia of tissues. Oxidation of the ferrous iron of hemoglobin to the ferric state produces methemoglobin.
Hundreds of different types of hemoglobin have been discovered. See: blood
hemoglobin A
hemoglobin A1c
Abbreviation: Hb A1cBarts hemoglobin
See: Barts hemoglobinhemoglobin C
hemoglobin E
fetal hemoglobin
Patient care
The induction of fetal hemoglobin (with drugs such as hydroxyurea) in patients with sickle cell anemia often improves their clinical status because fetal hemoglobin does not deform or “sickle” in the circulation. It is capable of taking up and giving off oxygen at lower oxygen tensions than the hemoglobin in adult erythrocytes.
free plasma hemoglobin
Plasma hemoglobin.glycated hemoglobin
Hemoglobin A1c.glycosylated hemoglobin
Hemoglobin A1c.hemoglobin Lepore
mean cell hemoglobin
mean corpuscular hemoglobin
Abbreviation: MCHMean cell hemoglobin.
plasma hemoglobin
Latest Searches:
Voraxaze - Voranil - Voorhoeve - voodoo - VOO - Vontrol - von - vomitus - vomiturition - vomitory - vomitoria - vomito - vomitive - vomiting - vomit - vomica - vomerovaginalis - vomerovaginal - vomerorostralis - vomerorostral -
- Service manuals - MBI Corp