Medical term:
Cipro
ciprofloxacin hydrochloride
Pharmacologic class: Fluoroquinolone
Therapeutic class: Anti-infective
Pregnancy risk category C
FDA Box Warning
• Fluoroquinolones for systemic use are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in patients usually over age 60, with concomitant use of corticosteroids, and in kidney, heart, and lung transplant recipients.
Action
Inhibits bacterial DNA synthesis by inhibiting DNA gyrase in susceptible gram-negative and gram-positive organisms
Availability
Injection: 200 mg/20 ml, 400 mg/40 ml, 200 mg/100 ml premixed in dextrose 5% in water (D5W), 400 mg/200 ml premixed in D5W, 1,200 mg/120-ml bulk package
Ophthalmic ointment: 3.5-g tube
Ophthalmic solution: 2.5-ml and 5-ml plastic dispensers
Otic solution: 0.2% (0.5 mg in 0.25 ml) in single-use container
Tablets: 250 mg, 500 mg, 750 mg
Indications and dosages
➣ Acute sinusitis
Adults: 500 mg P.O. q 12 hours or 400 mg I.V. q 12 hours for 10 days
➣ Prostatitis
Adults: 500 mg P.O. q 12 hours or 400 mg I.V. q 12 hours for 28 days
➣ Intra-abdominal infections
Adults: 500 mg P.O. q 12 hours or 400 mg I.V. q 12 hours for 7 to 14 days
➣ Febrile neutropenic patients
Adults: 400 mg I.V. q 8 hours for 7 to 14 days
➣ Gonorrhea
Adults: 500 mg P.O. as a single dose
➣ Infectious diarrhea
Adults: 500 mg P.O. q 12 hours for 5 to 7 days
➣ Inhalation anthrax (postexposure)
Adults: 500 mg P.O. q 12 hours for 60 days or 400 mg I.V. q 12 hours for 60 days
Children: 15 mg/kg P.O. q 12 hours for 60 days (not to exceed 500 mg/dose), or 10 mg/kg I.V. q 12 hours for 60 days, not to exceed 400 mg/dose
➣ Infections of lower respiratory tract, skin and skin structures, bones, and joints
Adults: 500 to 750 mg P.O. q 12 hours or 400 mg I.V. q 8 hours for 7 to 14 days. Severe bone and joint infections may necessitate up to 6 weeks of therapy.
➣ Nosocomial pneumonia
Adults: 400 mg I.V. q 8 hours for 10 to 14 days
➣ Typhoid fever
Adults: 500 mg P.O. q 12 hours for 10 days
➣ Urinary tract infections
Adults: 250 to 500 mg P.O. q 12 hours or 200 to 400 mg I.V. q 12 hours for 3 days in acute uncomplicated infection or for 7 to 14 days in acute complicated infection
➣ Complicated urinary tract infections or pyelonephritis
Children ages 1 to 17: 6 to 10 mg/kg I.V. q 8 hours for 10 to 21 days (maximum, 400 mg/dose; not to be exceeded, even in patients weighing more than 51 kg [112 lb]). Or, 10 to 20 mg/kg P.O. q 12 hours for 10 to 21 days (maximum, 750 mg/dose; not to be exceeded, even in patients weighing more than 51 kg).
➣ Acute otitis externa
Adults: Instill contents of one single-use otic solution container (0.5 mg) into affected ear b.i.d. (approximately 12 hours apart) for 7 days
➣ Bacterial conjunctivitis caused by susceptible organisms
Adults: 0.5″ ribbon of ophthalmic ointment applied to conjunctival sac t.i.d. on first 2 days, then 0.5" ribbon b.i.d. for 5 days. Or one to two drops of ophthalmic solution applied to conjunctival sac q 2 hours while awake for 2 days, then one or two drops q 4 hours while awake for 5 days.
➣ Corneal ulcers caused by susceptible organisms
Adults: Two drops of ophthalmic solution instilled into affected eye q 15 minutes for first 6 hours, then two drops into affected eye q 30 minutes for remainder of first day. On second day, two drops of ophthalmic solution hourly; on days 3 through 14, two drops q 4 hours.
Dosage adjustment
• Renal impairment or insufficiency
Off-label uses
• Chancroid
• Cystic fibrosis
• Pseudomembranous colitis caused by anti-infectives
Contraindications
• Hypersensitivity to drug or other fluoroquinolones
• Comcomitant administration of tizanidine
Precautions
Use cautiously in:
• cirrhosis, renal impairment, underlying CNS disease
• concurrent use of theophylline (risk of serious or fatal reactions, such as cardiac arrest, seizures, status epilepticus, and respiratory failure)
• elderly patients
• pregnant or breastfeeding patients
• children younger than age 18 (except for complicated urinary tract infection, pyelonephritis, and postexposure inhalation antrax only).
Administration
• Administer oral drug with or without food but not with dairy products or calcium-fortified juices alone; however, drug may be taken with a meal that contains these products.
• Infuse I.V. dose over at least 1 hour, using pump to ensure 1-hour duration.
☞ Know that too-rapid I.V. infusion increases risk of anaphylaxis and other adverse reactions.
☞ Know that treatment with ophthalmic solution may be continued after 14 days if corneal re-epithelialization hasn't occurred.
Adverse reactions
CNS: agitation, headache, restlessness, confusion, delirium, peripheral neuropathy, toxic psychosis
CV: orthostatic hypotension, vasculitis
EENT: nystagmus; with ophthalmic use-blurred vision; burning, stinging, irritation, itching, tearing, and redness of eyes; eyelid itching, swelling, or crusting; sensitivity to light
GI: nausea, vomiting, diarrhea, constipation, abdominal pain or discomfort, dyspepsia, dysphagia, flatulence, pancreatitis, pseudomembranous colitis
GU: albuminuria, candiduria, renal calculi
Hematologic: methemoglobinemia, agranulocytosis, hemolytic anemia
Hepatic: jaundice, hepatic necrosis
Metabolic: hyperglycemia, hyperkalemia
Musculoskeletal: myalgia, myoclonus, tendinitis, tendon rupture
Skin: rash, exfoliative dermatitis, toxic epidermal necrolysis, erythema multiforme photosensitivity
Other: injection-site reaction, altered taste, anosmia, exacerbation of myasthenia gravis, overgrowth of nonsusceptible organisms, hypersensitivity reactions including anaphylaxis and Stevens-Johnson syndrome
Interactions
Drug-drug. Antacids, bismuth subsalicylate, iron salts, sucralfate, zinc salts: decreased ciprofloxacin absorption
Cyclosporine: transient creatinine increase
Hormonal contraceptives: reduced contraceptive efficacy
Oral anticoagulants: increased anticoagulant effects
Phenytoin: increased or decreased phenytoin blood level
Probenecid: decreased renal elimination of ciprofloxacin, causing increased blood level
Theophylline: increased theophylline blood level, greater risk of toxicity
Tizanidine: significantly elevated tizanidine plasma level
Drug-diagnostic tests. Alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, bilirubin, cholesterol, glucose, lactate dehydrogenase, potassium, triglycerides: increased levels Prothrombin time: prolonged
Drug-food. Caffeine: interference with caffeine clearance
Concurrent tube feedings, milk or yogurt (when consumed alone with ciprofloxacin): impaired drug absorption
Drug-herbs. Fennel: decreased drug absorption
Patient monitoring
• In patients with renal insufficiency, assess creatinine level before giving first dose and at least once a week during prolonged therapy. Monitor drug blood level closely.
☞ Watch for signs and symptoms of serious adverse reactions, including GI problems, jaundice, tendon problems, and hypersensitivity reactions.
Patient teaching
• Tell patient to take drug with or without food at the same time each day.
• Advise patient not to take drug with dairy products or calcium-fortified juices alone or with caffeinated beverages.
• Advise patient to drink 8 oz of water every hour while awake to ensure adequate hydration.
☞ Instruct patient to stop taking drug and notify prescriber at first sign of burning, numbness, or tingling in hands or feet; yellow eyes or skin; unusual tiredness; persistent diarrhea; rash; or tendon pain, swelling, or inflammation.
• Advise patient to avoid excessive exposure to sun or ultraviolet light and to discontinue drug and notify prescriber if phototoxicity (burning, erythema, exudation, vesicles, blistering, edema) occurs.
• Advise patient taking hormonal contraceptives to use supplemental birth control method, such as condoms, because drug reduces contraceptive efficacy.
• Inform breastfeeding patient that drug is excreted in breast milk and can affect infant's bone growth. Advise her to consult prescriber before using drug.
• Teach patient how to use eye ointment or solution and tell patient not to touch eye dropper tip to any surface, to avoid contamination.
• Instruct patient how to use ear solution and to lie with affected ear upward for at least 1 minute after instilling solution.
• Caution patient with bacterial conjunctivitis not to wear contact lenses.
• As appropriate, review all other significant and life-threatening adverse reactions and interactions, especially those related to the drugs, tests, foods, and herbs mentioned above.
ciprofloxacin
(sip-roe-flox-a-sin) ,Cipro
(trade name),Cipro XR
(trade name)Classification
Therapeutic: anti infectivesPharmacologic: fluoroquinolones
Indications
- Urinary tract and gynecologic infections, including cystitis, and prostatitis,
- Respiratory tract infections including acute sinusitis, acute exacerbations of chronic bronchitis, and pneumonia,
- Skin and skin structure infections,
- Bone and joint infections,
- Infectious diarrhea,
- Complicated intra-abdominal infections (with metronidazole),
- Typhoid fever.
Action
Therapeutic effects
- Staphylococcus aureus,
- Staphylococcus epidermidis,
- Staphylococcus saprophyticus,
- Streptococcus pyogenes,
- Streptococcus pneumoniae,
- Enterococcus faecalis,
- Bacillus anthracis (anthrax).
- Escherichia coli,
- Klebsiella pneumoniae,
- Enterobacter cloacae,
- Salmonella typhi,
- Shigella spp,
- Proteus mirabilis,
- Proteus vulgaris,
- Providencia stuartii,
- Providencia rettgeri,
- Morganella morganii,
- Pseudomonas aeruginosa,
- Serratia marcescens,
- Haemophilus influenzae,
- ,
- Moraxella catarrhalis,
- Campylobacter jejuni.
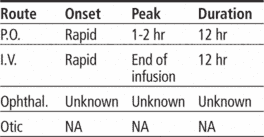
Pharmacokinetics
Time/action profile (blood levels)
| ROUTE | ONSET | PEAK | DURATION |
| PO | rapid | 1–2 hr | 12 hr |
| PO-ER | rapid | 1–4 hr | 24 hr |
| IV | rapid | end of infusion | 12 hr |
Contraindications/Precautions
Adverse Reactions/Side Effects
Central nervous system
- elevated intracranial pressure (including pseudotumor cerebri) (life-threatening)
- seizures (life-threatening)
- agitation
- confusion
- depression
- dizziness
- drowsiness
- hallucinations
- headache
- insomnia
- nightmares
- paranoia
- tremor
Gastrointestinal
- hepatotoxicity (life-threatening)
- pseudomembranous colitis (life-threatening)
- abdominal pain
- diarrhea (most frequent)
- nausea (most frequent)
- ↑ liver enzymes
Genitourinary
- vaginitis
Dermatologic
- photosensitivity
- rash
Endocrinologic
- hyperglycemia
- hypoglycemia
Hematologic
- eosinophilia
Local
- phlebitis at IV site
Musculoskeletal
- tendinitis
- tendon rupture
Neurologic
- peripheral neuropathy
Miscellaneous
- hypersensitivity reactions including :
- anaphylaxis (life-threatening)
Interactions
Drug-Drug interaction
Concurrent use with theophylline may result in ↑ theophylline concentrations and therefore serious and potentially fatal reactions due to theophylline toxicity; if concurrent use cannot be avoided serum theophylline levels should be monitored.Administration with antacids, iron salts, bismuth subsalicylate, sucralfate, and zinc salts ↓ absorption.May alter the effects of warfarin.May ↓ levels and effectiveness of phenytoin.Serum levels may be ↓ by antineoplastics .Cimetidine may interfere with elimination.Beneficial effects may be antagonized by nitrofurantoin.Probenecid ↓ renal elimination.May ↑ risk of nephrotoxicity from cyclosporine.Concurrent use with foscarnet may ↑ risk of seizures.Concurrent therapy with corticosteroids may ↑ risk of tendon rupture.May ↓ metabolism of tizanidine, use contraindicated.Fennel ↓ bioavailability.Absorption is impaired by concurrent enteral feeding (because of metal cations).Absorption is ↓ by food and/or dairy products (take 1 hr before or 2 hr after).Route/Dosage
Most infections
Renal Impairment
Oral (Adults) CCr 30–50 mL/min—250–500 mg q 12 hr; CCr 5–29 mL/min—250–500 mg q 18 hr; Hemodialysis or peritoneal dialysis—250–500 mg q 24 hr.Renal Impairment
Intravenous (Adults) CCr 5–29 mL/min—200–400 mg q 18–24 hr.Urinary tract infections
Inhalational Anthrax
Cutaneous anthrax
Cystic Fibrosis
Availability (generic available)
Nursing implications
Nursing assessment
- Assess for infection (vital signs; appearance of wound, sputum, urine, and stool; WBC; urinalysis; frequency and urgency of urination; cloudy or foul-smelling urine) at beginning of and throughout therapy.
- Obtain specimens for culture and sensitivity before initiating therapy. First dose may be given before receiving results.
- Observe for signs and symptoms of anaphylaxis (rash, pruritus, laryngeal edema, wheezing). Discontinue drug and notify health care professional immediately if these problems occur. Keep epinephrine, an antihistamine, and resuscitation equipment close by in case of an anaphylactic reaction.
- Monitor bowel function. Diarrhea, abdominal cramping, fever, and bloody stools should be reported to health care professional promptly as a sign of pseudomembranous colitis. May begin up to several weeks following cessation of therapy.
- Lab Test Considerations: .
- May cause ↑ serum AST, ALT, LDH, bilirubin, and alkaline phosphatase.
- May also cause ↑ or ↓ serum glucose.
Potential Nursing Diagnoses
Risk for infection (Patient/Family Teaching)Implementation
- Oral: .
- Administer on an empty stomach 1 hr before or 2 hr after meals, with a full glass of water. Products or foods containing calcium, magnesium, aluminum, iron, or zinc should not be ingested for 4 hr before and 2 hr after administration.
- If gastric irritation occurs, ciprofloxacin may be administered with meals. Food slows and may slightly decrease absorption.
- Regular tablets can be crushed for patients unable to swallow. Extended-release (XR) tablets should be swallowed whole; do not split, crush, or chew.
- Do not administer 5% or 10% oral solution through an enteral feeding tube or with enteral feedings; may decrease absorption.
Intravenous Administration
- pH: 3.3–4.6.
- Intermittent Infusion: Diluent: Dilute with 0.9% NaCl or D5W. Stable for 14 days at refrigerated or room temperature. Concentration: 1–2 mg/mL.
- Rate: Administer over 60 min into a large vein to minimize venous irritation.
- Y-Site Compatibility: alemtuzumab, amifostine, amiodarone, anakinra, anidulafungin, argatroban, aztreonam, bivalirudin, bleomycin, calcium gluconate, carboplatin, carmustine, caspofungin, ceftaroline, ceftazidime, cisatracurium, cisplatin, cyclophosphamide, cytarabine, dactinomycin, daptomycin, dexmedetomidine, dexrazoxane, digoxin, diltiazem, diphenhydramine, dobutamine, docetaxel, dolasetron, dopamine, doripenem, doxacurium, doxorubicin, doxorubicin liposome, epirubicin, eptifibatide, ertapenem, etoposide, etoposide phosphate, fenoldopam, fludarabine, gemcitabine, gentamicin, granisetron, hetastarch, hydromorphone, idarubicin, ifosfamide, irinotecan, leucovorin calcium, lidocaine, linezolid, lorazepam, mechlorethamine, meperidine, methotrexate, metoclopramide, metronidazole, midazolam, midodrine, milrinone, mitoxantrone, mycophentolate, nesiritide, octreotide, ondansetron, oxaliplatin, oxytocin, paclitaxel, palonosetron, pamidronate, pancuronium, potassium acetate, potassium chloride, promethazine, quinupristin-dalfopristin, ranitidine, remifentanil, rocuronium, sodium acetate, tacrolimus, telavancin, teniposide, thiotepa, tigecycline, tirofiban, tobramycin, trastuzumab, vancomycin, vasopressin, vecuronium, verapamil, vinblastine, vincristine, vinorelbine, voriconazole, zoledronic acid
- Y-Site Incompatibility: Manufacturer recommends temporarily discontinuing other solutions when administering ciprofloxacin.acyclovir, aminocaproic acid, aminophylline, amphotericin B lipid complex, amphotericin B liposome, ampicillin/sulbactam, cefepime, dexamethasone, fluorouracil, foscarnet, furosemide, heparin, hydrocortisone, magnesium sulfate, methylprednisolone, pantoprazole, pemetrexed, phenytoin, piperacillin/tazobactam, potassium phosphates, propofol, rituxumab, sodium phosphates, warfarin
Patient/Family Teaching
- Instruct patient to take medication as directed at evenly spaced times and to finish drug completely, even if feeling better. Take missed doses as soon as possible, unless almost time for next dose. Do not double doses. Advise patient that sharing of this medication may be dangerous.
- Advise patients to notify health care professional immediately if they are taking theophylline.
- Encourage patient to maintain a fluid intake of at least 1500–2000 mL/day to prevent crystalluria.
- Advise patient that antacids or medications containing calcium, magnesium, aluminum, iron, or zinc will decrease absorption and should not be taken within 4 hr before and 2 hr after taking this medication.
- May cause dizziness and drowsiness. Caution patient to avoid driving or other activities requiring alertness until response to medication is known.
- Caution patient to use sunscreen and protective clothing to prevent phototoxicity reactions during and for 5 days after therapy. Notify health care professional if a sunburn-like reaction or skin eruption occurs.
- Instruct patients being treated for gonorrhea that partners also must be treated.
- Instruct patient to notify health care professional of all Rx or OTC medications, vitamins, or herbal products being taken and to consult health care professional before taking any other Rx, OTC, or herbal products.
- Advise patient to report signs of superinfection (furry overgrowth on the tongue, vaginal itching or discharge, loose or foul-smelling stools).
- Instruct patient to notify health care professional if fever and diarrhea develop, especially if stool contains blood, pus, or mucus. Advise patient not to treat diarrhea without consulting health care professional.
- Instruct patient to notify health care professional immediately if signs and symptoms of hepatotoxicity (anorexia, jaundice, dark urine, pruritus, or tender abdomen), rash, signs of hypersensitivity, or tendon (shoulder, hand, Achilles, and other) pain, swelling, or inflammation occur. If tendon symptoms occur, avoid exercise and use of the affected area. Increased risk in >65 yrs old, kidney, heart and lung transplant recipients, and patients taking corticosteroids concurrently. Therapy should be discontinued.
Evaluation/Desired Outcomes
- Resolution of the signs and symptoms of bacterial infection. Time for complete resolution depends on organism and site of infection.
- Post-exposure treatment of inhalational anthrax or cutaneous anthrax.
Cipro
(sĭp′rō)Latest Searches:
Voraxaze - Voranil - Voorhoeve - voodoo - VOO - Vontrol - von - vomitus - vomiturition - vomitory - vomitoria - vomito - vomitive - vomiting - vomit - vomica - vomerovaginalis - vomerovaginal - vomerorostralis - vomerorostral -
- Service manuals - MBI Corp