Medical term:
Haldol
Haldol
[hal´dol]haloperidol
haloperidol decanoate
haloperidol lactate
Pharmacologic class: Butyrophenone
Therapeutic class: Antipsychotic
Pregnancy risk category C
FDA Box Warning
Action
Unknown. Thought to block postsynaptic dopamine receptors in brain and increase dopamine turnover rate, inhibiting signs and symptoms of psychosis.
Availability
Injection (decanoate): 50 mg/ml, 100 mg/ml
Injection (lactate): 5 mg/ml
Oral concentrate (lactate): 2 mg/ml
Tablets: 0.5 mg, 1 mg, 2 mg, 5 mg, 10 mg, 20 mg
Indications and dosages
➣ Symptomatic treatment of psychotic disorders or Tourette syndrome
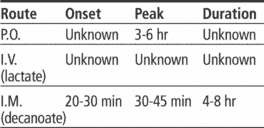
Adults: For moderate symptoms, 0.5 to 2 mg P.O. two to three times daily. For severe symptoms or chronic or resistant disorder, 3 to 5 mg P.O. two to three times daily, to a maximum of 100 mg daily if needed. Adjust subsequent dosages carefully based on response and tolerance. Alternatively, 2 to 5 mg I.M. (lactate) may be given for prompt control of acutely agitated patient with moderate to severe symptoms; based on response, subsequent doses may be given q hour.
➣ Schizophrenia in patients who need prolonged parenteral antipsychotic therapy
Adults: For patient previously stabilized on oral haloperidol, initial I.M. dose (decanoate) is 10 to 20 times the previous daily P.O. haloperidol equivalent, depending on patient's stability on low or high P.O. dosage. Initially, I.M. dosage shouldn't exceed 100 mg. If conversion requires dosage above 100 mg, give balance in 3 to 7 days. Maintenance dosage is 10 to 15 times the previous daily P.O. dosage, depending on response.
➣ Psychotic disorders
Children ages 3 to 12 or weighing 15 to 40 kg (33 to 88 lb): 0.05 to 0.15 mg/kg/day P.O. in two or three divided doses. May be increased by 0.5 mg daily given in two or three divided doses at 5- to 7-day intervals, depending on response and tolerance.
➣ Nonpsychotic behavior disorder; Tourette syndrome; hyperactivity
Children ages 3 to 12 or weighing 15 to 40 kg (33 to 88 lb): 0.05 to 0.075 mg/kg/day P.O. in two or three divided doses
Dosage adjustment
• Elderly or debilitated patients
Off-label uses
• Nausea and vomiting
• Infantile autism
• Intractable hiccups
Contraindications
• Hypersensitivity to drug, tartrazine, sesame oil, or benzyl alcohol (with some products)
• Severe CNS depression or comatose states
• Parkinson's disease
Precautions
Use cautiously in:
• torsades de pointes, QT interval prolongation
• patients with allergies
• hepatic disease, bone marrow depression, cardiac disease, respiratory insufficiency, CNS tumors
• history of seizures, patients receiving anticonvulsants, EEG abnormalities
• concurrent anticoagulant use
• elderly patients
• pregnant or breastfeeding patients
• children (parenteral form not recommended).
Administration
• Be aware that torsades de pointes and QT interval prolongation have occurred in patients receiving haloperidol, especially when drug is given I.V. or in doses higher than recommended. Haloperidol isn't approved for I.V. use.
☞ Don't give decanoate form I.V.
• Administer decanoate form by deep I.M. injection using 21G needle. Two injections may be necessary; maximum volume shouldn't exceed 3 ml.
• Know that recommended interval between I.M. injections is 4 weeks.
• Dilute oral concentrate in water, soda, or juice (orange, apple, tomato) immediately before administering.
• Be aware that patient should be switched from parenteral form to oral form as soon as possible.
• Know that parenteral form is not recommended in children.
Adverse reactions
CNS: confusion, drowsiness, restlessness, extrapyramidal reactions, sedation, lethargy, insomnia, vertigo, tardive dyskinesia, seizures, neuroleptic malignant syndrome
CV: hypotension, hypertension, tachycardia, ECG changes, torsades de pointes (with I.V. use)
EENT: blurred vision, dry eyes
GI: constipation, ileus, dry mouth, anorexia
GU: urinary retention, menstrual irregularities, gynecomastia, priapism
Hematologic: anemia, leukocytosis, leukopenia
Hepatic: jaundice, drug-induced hepatitis
Metabolic: galactorrhea
Respiratory: dyspnea, respiratory depression, bronchospasm, laryngospasm
Skin: diaphoresis, photosensitivity, rash
Other: hyperpyrexia, hypersensitivity reactions
Interactions
Drug-drug. Antidepressants, antihistamines, atropine, disopyramide, phenothiazines, quinidine, other anticholinergics: additive anticholinergic effects
Antihypertensives, nitrates: additive hypotension
CNS depressants (including antihistamines, opioid analgesics, sedative-hypnotics): additive CNS depression
Epinephrine: severe hypotension and tachycardia
Levodopa, pergolide: decreased therapeutic effects of haloperidol
Lithium: acute encephalopathic syndrome
Methyldopa: dementia
Rifampin: decreased haloperidol plasma level
Drug-diagnostic tests. Alanine aminotransferase, aspartate aminotransferase, thyroid function tests: increased values
Arterial blood gases, bicarbonate: altered values
White blood cells: increased or decreased count
Drug-herbs. Angel's trumpet, jimsonweed, scopolia: antagonism of cholinergic effects
Chamomile, hops, kava, skullcap, valerian: increased CNS depression
Nutmeg: reduced haloperidol efficacy
Drug-behaviors. Acute alcohol ingestion: additive hypotension
Patient monitoring
☞ Monitor CNS status closely, especially for seizures and neuroleptic malignant syndrome (shown by extrapyramidal symptoms, hyperthermia, and autonomic disturbances).
☞ Monitor cardiovascular status, particularly for ECG changes, blood pressure changes, torsades de pointes, and atypical rapid ventricular tachycardia, which may progress to ventricular fibrillation (with I.V. use).
• Assess respiratory status.
• Monitor liver function test results and CBC with white cell differential.
• With prolonged use, assess for tardive dyskinesia (which may occur months or even years after starting drug).
Patient teaching
• Tell patient to dilute oral concentrate with water, cola, or juice immediately before taking.
☞ Instruct patient to immediately report signs or symptoms of serious adverse reactions, such as unusual weakness, yellowing of skin or eyes, difficulty breathing, or symptoms of neuroleptic malignant syndrome (such as fever, muscle pain or rigidity, rapid or irregular pulse, increased sweating, change in urination pattern, or decreased mental acuity).
• Advise patient to minimize GI upset by eating frequent, small servings of food and drinking adequate fluids.
• As appropriate, review all other significant and life-threatening adverse reactions and interactions, especially those related to the drugs, tests, herbs, and behaviors mentioned above.
Haldol
(hăl′dôl′, -dŏl′, -dōl′)Haldol®
Haloperidol, see there.Haldol
A brand name for HALOPERIDOL.Haldol
A brand name for HALOPERIDOL.Latest Searches:
Voraxaze - Voranil - Voorhoeve - voodoo - VOO - Vontrol - von - vomitus - vomiturition - vomitory - vomitoria - vomito - vomitive - vomiting - vomit - vomica - vomerovaginalis - vomerovaginal - vomerorostralis - vomerorostral -
- Service manuals - MBI Corp