Medical term:
Humalog
insulin, regular (insulin injection)
insulin (lispro)
insulin glulisine, recombinant
insulin lispro protamine, human
isophane insulin suspension (NPH insulin)
isophane insulin suspension (NPH) and insulin injection (regular)
Pharmacologic class: Pancreatic hormone
Therapeutic class: Hypoglycemic
Pregnancy risk category B
Action
Promotes glucose transport, which stimulates carbohydrate metabolism in skeletal and cardiac muscle and adipose tissue. Also promotes phosphorylation of glucose in liver, where it is converted to glycogen. Directly affects fat and protein metabolism, stimulates protein synthesis, inhibits release of free fatty acids, and indirectly decreases phosphate and potassium.
Availability
Glulisine, recombinant: 100 units/ml in 10-ml vials, 100 units/ml in 3-ml cartridge system, 100 units/ml in 3-ml prefilled pen
Isophane suspension, injection (regular): 70 units NPH and 30 units regular insulin/ml (100 units/ml total), 50 units NPH and 50 units regular insulin/ml (100 units/ml total)
Isophane suspension (NPH insulin): 100 units/ml
Lispro: 100 units/ml in 10-ml vials and 1.5-ml cartridges
Regular insulin injection: 100 units/ml
Regular U-500 (concentrated), insulin human injection: 500 units/ml
Zinc suspension, extended (ultralente): 100 units/ml
Zinc suspension (lente insulin): 100 units/ml
Indications and dosages
➣ Type 1 (insulin-dependent) diabetes mellitus; type 2 (non-insulin-dependent) diabetes mellitus unresponsive to diet and oral hypoglycemics
Adults and children: In newly diagnosed diabetes, total of 0.5 to 1 unit/kg/day subcutaneously as part of multidose regimen of short- and long-acting insulin. Dosage individualized based on patient's glucose level, adjusted to premeal and bedtime glucose levels. Reserve concentrated insulin (500 units/ml) for patients requiring more than 200 units/day.
➣ Diabetic ketoacidosis
Adults and children: Loading dose of 0.15 units/kg (nonconcentrated regular insulin) I.V. bolus, followed by continuous infusion of 0.1 unit/kg/hour until glucose level drops. Then administer subcutaneously, adjusting dosage according to glucose level.
Contraindications
• Hypersensitivity to drug or its components
• Hypoglycemia
Precautions
Use cautiously in:
• hepatic or renal impairment, hypothyroidism, hyperthyroidism
• elderly patients
• pregnant or breastfeeding patients
• children.
Administration
☞ Be aware that insulin is a high-alert drug whether given subcutaneously or I.V.
☞ Don't give insulin I.V. (except nonconcentrated regular insulin), because anaphylactic reaction may occur.
• When mixing two types of insulin, draw up regular insulin into syringe first.
• For I.V. infusion, mix regular insulin only with normal or half-normal saline solution, as prescribed, to yield a concentration of 1 unit/ml. Give every 50 units I.V. over at least 1 minute.
• Rotate subcutaneous injection sites to prevent lipodystrophy.
• Administer mixtures of regular and NPH or regular and lente insulins within 5 to 15 minutes of mixing.
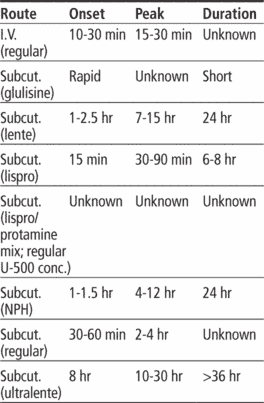
Adverse reactions
Metabolic: hypokalemia, sodium retention, hypoglycemia, rebound hyperglycemia (Somogyi effect)
Skin: urticaria, rash, pruritus
Other: edema; lipodystrophy; lipohypertrophy; erythema, stinging, or warmth at injection site; allergic reactions including anaphylaxis
Interactions
Drug-drug. Acetazolamide, albuterol, antiretrovirals, asparaginase, calcitonin, corticosteroids, cyclophosphamide, danazol, dextrothyroxine, diazoxide, diltiazem, diuretics, dobutamine, epinephrine, estrogens, hormonal contraceptives, isoniazid, morphine, niacin, phenothiazines, phenytoin, somatropin, terbutaline, thyroid hormones: decreased hypoglycemic effect
Anabolic steroids, angiotensin-converting enzyme inhibitors, calcium, chloroquine, clofibrate, clonidine, disopyramide, fluoxetine, guanethidine, mebendazole, MAO inhibitors, octreotide, oral hypoglycemics, phenylbutazone, propoxyphene, pyridoxine, salicylates, sulfinpyrazone, sulfonamides, tetracyclines: increased hypoglycemic effect
Beta-adrenergic blockers (nonselective): masking of some hypoglycemia symptoms, delayed recovery from hypoglycemia
Lithium carbonate: decreased or increased hypoglycemic effect
Pentamidine: increased hypoglycemic effect, possibly followed by hyperglycemia
Drug-diagnostic tests. Glucose, inorganic phosphate, magnesium, potassium: decreased levels
Liver and thyroid function tests: interference with test results
Urine vanillylmandelic acid: increased level
Drug-herbs. Basil, burdock, glucosamine, sage: altered glycemic control Chromium, coenzyme Q10, dandelion, eucalyptus, fenugreek, marshmallow: increased hypoglycemic effect
Garlic, ginseng: decreased blood glucose level
Drug-behaviors. Alcohol use: increased hypoglycemic effect
Marijuana use: increased blood glucose level
Smoking: increased blood glucose level, decreased response to insulin
Patient monitoring
• Monitor glucose level frequently to assess drug efficacy and appropriateness of dosage.
• Watch blood glucose level closely if patient is converting from one insulin type to another or is under unusual stress (as from surgery or trauma).
☞ Monitor for signs and symptoms of hypoglycemia. Keep glucose source at hand in case hypoglycemia occurs.
☞ Assess for signs and symptoms of hyperglycemia, such as polydipsia, polyphagia, polyuria, and diabetic ketoacidosis (as shown by blood and urinary ketones, metabolic acidosis, extremely elevated blood glucose level).
• Monitor for glycosuria.
• Closely evaluate kidney and liver function test results in patients with renal or hepatic impairment.
Patient teaching
• Teach patient how to administer insulin subcutaneously as appropriate.
• Advise patient to draw up regular insulin into syringe first when mixing two types of insulin. Caution him not to change order of mixing insulins.
• Instruct patient to rotate subcutaneous injection sites and keep a record of sites used, to prevent fatty tissue breakdown.
☞ Teach patient how to recognize and report signs and symptoms of hypoglycemia and hyperglycemia. Advise him to carry a glucose source at all times.
• Instruct patient to store insulin in refrigerator (not freezer).
• Teach patient how to monitor and record blood glucose level and, if indicated, urine glucose and ketone levels.
• Tell patient that dietary changes, activity, and stress can alter blood glucose level and insulin requirements.
• Instruct patient to wear medical identification stating that he is diabetic and takes insulin.
• Advise patient to have regular medical, vision, and dental exams.
• As appropriate, review all other significant and life-threatening adverse reactions and interactions, especially those related to the drugs, tests, herbs, and behaviors mentioned above.
insulin lispro, rDNA origin
,HumaLOG
(trade name)insulin lispro protamine suspension/insulin lispro injection mixtures, rDNA origin
,HumaLOG Mix 75/25
(trade name),HumaLOG Mix 50/50
(trade name)Classification
Therapeutic: antidiabeticsPharmacologic: pancreatics
See for more information concerning insulins
Indications
Action
- stimulating glucose uptake in skeletal muscle and fat,
- inhibiting hepatic glucose production.
- inhibition of lipolysis and proteolysis,
- enhanced protein synthesis.
Therapeutic effects
Pharmacokinetics
Time/action profile (hypoglycemic effect)
| ROUTE | ONSET | PEAK | DURATION |
|---|---|---|---|
| Insulin lispro subcut | within 15 min | 1–2 hr | 3–4 hr |
| 75% insulin lispro protamine suspension/25% insulin lispro injection subcut | within 15 min | 2.8 hr | 24 hr |
Contraindications/Precautions
Adverse Reactions/Side Effects
Endocrinologic
- hypoglycemia (life-threatening)
Local
- lipodystrophy
- pruritus
- erythema
- swelling
Miscellaneous
- allergic reactions including anaphylaxis (life-threatening)
Interactions
Drug-Drug interaction
Beta blockers, clonidine, and reserpine may mask some of the signs and symptoms of hypoglycemia.Corticosteroids, thyroid supplements, estrogens, isoniazid, niacin,phenothiazines,, and rifampin may ↑ insulin requirements.Alcohol, ACE inhibitors, MAO inhibitors, octreotide, oral hypoglycemic agents, and salicylates, may ↓ insulin requirements.Concurrent use with pioglitazone or rosiglitazone may ↑ risk of fluid retention and worsening HFGlucosamine may worsen blood glucose control.Fenugreek, chromium, and coenzyme Q-10 may produce additive hypoglycemic effects.Route/Dosage
Dose depends on blood glucose, response, and many other factorsAvailability
Nursing implications
Nursing assessment
- Assess for symptoms of hypoglycemia (anxiety; restlessness; tingling in hands, feet, lips, or tongue; chills; cold sweats; confusion; cool, pale skin; difficulty in concentration; drowsiness; nightmares or trouble sleeping; excessive hunger; headache; irritability; nausea; nervousness; tachycardia; tremor; weakness; unsteady gait)and hyperglycemia (confusion, drowsiness; flushed, dry skin; fruit-like breath odor; rapid, deep breathing, polyuria; loss of appetite; unusual thirst) periodically during therapy.
- Monitor body weight periodically. Changes in weight may necessitate changes in insulin dose.
- Lab Test Considerations:
- Monitor blood glucose every 6 hr during therapy, more frequently in ketoacidosis and times of stress. A1C may be monitored every 3–6 mo to determine effectiveness.
Overdose is manifested by symptoms of hypoglycemia. Mild hypoglycemia may be treated by ingestion of oral glucose. Severe hypoglycemia is a life-threatening emergency; treatment consists of IV glucose, glucagon, or epinephrine.
Potential Nursing Diagnoses
Noncompliance (Patient/Family Teaching)Implementation
- high alert: Medication errors involving insulins have resulted in serious patient harm and death. Clarify all ambiguous orders and do not accept orders using the abbreviation “u” for units, which can be misread as a zero or the numeral 4 and has resulted in tenfold overdoses. Insulins are available in different types and strengths. Check type, dose, and expiration date with another licensed nurse. Do not interchange insulins without consulting physician or other health care professional.
- Do not confuse Humalog with Humulin.
- Due to the short duration of action, insulin lispro must be used with a longer acting insulin, insulin infusion pump or in combination with oral sulfonurea agents.
- Use only insulin syringes to draw up dose. The unit markings on the insulin syringe must match the insulin’s units/mL. Special syringes for doses <50 units are available. Use only U-100 insulin syringes to draw up insulin lispro dose. Prior to withdrawing dose, rotate vial between palms to ensure uniform solution; do not shake.
- When mixing insulins, draw insulin lispro into syringe first to avoid contamination of regular insulin vial.
- Insulin should be stored in a cool place but does not need to be refrigerated.
- Subcutaneous: Administer insulin lispro within 15 min before a meal. Rotate injection sites. Do not administer IV.
- May be administered via disposable external insulin pump. Do not administer solution that appears thickened, cloudy, discolored or contains particles. Store cartridges for pump in refrigerator. Do not mix with other insulins or solutions when used with pump. Choose a new infusion site and insertion site at least every 3 days. Discard cartridges after 7 days, even if solution remains.
- Syringe Compatibility: May be diluted with sterile diluent for Humalog, Humulin N, Humulin 50/50, Humulin 70/30, and NPH Ilentin to a concentration of 1:10 or 1:2.
Intravenous Administration
- Continuous Infusion: Administer IV under medical supervision with close monitoring of blood glucose and potassium levels to avoid hypoglycemia and hypokalemia. Concentration: 0.1 unit/mL to 1.0 unit/mL. Diluent: 0.9% NaCl. Solutions of insulin lispro and 0.9% NaCl can be stored for 48 hrs in refrigerator, then used at room temperature for another 48 hr.
Patient/Family Teaching
- Instruct patient on proper technique for administration. Include type of insulin, equipment (syringe, cartridge pens, external pumps, alcohol swabs), storage, and place to discard syringes. Discuss the importance of not changing brands of insulin or syringes, selection and rotation of injection sites, and compliance with therapeutic regimen.
- Demonstrate technique for mixing insulins by drawing up insulin lispro first and rolling intermediate-acting insulin vial between palms to mix, rather than shaking (may cause inaccurate dose).
- Explain to patient that this medication controls hyperglycemia but does not cure diabetes. Therapy is long term.
- Instruct patient in proper testing of serum glucose and ketones. These tests should be closely monitored during periods of stress or illness and health care professional notified of significant changes.
- Emphasize the importance of compliance with nutritional guidelines and regular exercise as directed by health care professional.
- Advise patient to notify health care professional of all Rx or OTC medications, vitamins, or herbal products being taken and to consult with health care professional before taking other medications or alcohol.
- Advise patient to notify health care professional of medication regimen prior to treatment or surgery.
- Advise patient to notify health care professional if nausea, vomiting, or fever develops, if unable to eat regular diet, or if blood glucose levels are not controlled.
- Instruct patient on signs and symptoms of hypoglycemia and hyperglycemia and what to do if they occur.
- Advise patient to notify health care professional if pregnancy is planned or suspected or if breastfeeding or planning to breastfeed.
- Patients with diabetes mellitus should carry a source of sugar (candy, glucose gel) and identification describing their disease and treatment regimen at all times.
- Emphasize the importance of regular follow-up, especially during first few weeks of therapy.
Evaluation/Desired Outcomes
- Control of blood glucose levels in diabetic patients without the appearance of hypoglycemic or hyperglycemic episodes.
Humalog
(hyo͞o′mə-lôg′, -lŏg′)Humalog
A brand name for INSULIN LISPRO.Latest Searches:
Voraxaze - Voranil - Voorhoeve - voodoo - VOO - Vontrol - von - vomitus - vomiturition - vomitory - vomitoria - vomito - vomitive - vomiting - vomit - vomica - vomerovaginalis - vomerovaginal - vomerorostralis - vomerorostral -
- Service manuals - MBI Corp