Medical term:
Opana
oxymorphone hydrochloride
Pharmacologic class: Opioid agonist
Therapeutic class: Narcotic analgesic
Controlled substance schedule II
Pregnancy risk category C
FDA Box Warning
• Drug is morphine-like opioid agonist and Schedule II controlled substance, with abuse potential similar to other opioids. This potential must be considered when prescribing or dispensing drug.
• Drug is indicated for managing moderate to severe pain when continuous, around-the-clock opioid is needed for extended period. It isn't intended for as-needed analgesia.
• Instruct patients to swallow extended-release tablets whole. Caution them not to break, chew, dissolve, or crush them, as this causes rapid release and absorption of potentially fatal dose.
• Caution patient not to consume alcoholic beverages or take prescription or nonprescription medications containing alcohol during therapy, as this may increase drug blood levels and cause potentially fatal overdose.
Action
Unknown. Thought to interact with opioid receptor sites primarily in limbic system, thalamus, and spinal cord, blocking pain impulse transmission.
Availability
Injection: 1 mg/ml, 1.5 mg/ml
Tablets: 5 mg, 10 mg
Tablets (extended-release): 5 mg, 7.5 mg, 10 mg, 15 mg, 20 mg, 30 mg, 40 mg
Indications and dosages
➣ Moderate to severe pain
Adults: 1 to 1.5 mg I.M. or subcutaneously q 4 to 6 hours p.r.n.; or initially, 0.5 mg I.V., increased cautiously until pain relief is satisfactory
➣ To reduce labor pain
Adults: 0.5 to 1 mg I.M.
➣ Initiation of therapy for moderate to severe acute pain in opioid-naïve patients
Adults: 10 to 20 mg (Opana) P.O. q 4 to 6 hours depending on initial pain intensity. If deemed necessary to initiate therapy at lower dose, start with 5 mg. Adjust dosage based on patient's response to initial dose. Dose can then be adjusted to acceptable level of analgesia taking into account pain intensity and adverse effects experienced. For chronic around-the-clock opioid therapy, give 5 mg (Opana ER) q 12 hours; thereafter, individually adjust dosage, preferably at increments of 5 to 10 mg q 12 hours every 3 to 7 days to level that provides adequate analgesia and minimizes side effects; give under close supervision of prescribing physician.
➣ Moderate to severe acute pain when converting from parenteral to oral form in patients requiring continuous, around-the-clock opioid treatment for extended period
Adults: 10 times patient's total daily parenteral oxymorphone dose as Opana, in four or six equally divided doses (for example, approximately 10 mg Opana may be needed to provide pain relief equivalent to total daily I.M. dose of 4 mg oxymorphone); titrate dosage to optimal pain relief or combine with acetaminophen/nonsteroidal anti-inflammatories for optimal pain relief. Or 10 times patient's total daily parenteral oxymorphone dose as Opana ER, in two equally divided doses (for example, approximately 20 mg Opana ER q 12 hours may be needed to provide pain relief equivalent to total daily parenteral dose of 4 mg oxymorphone.
➣ Moderate to severe acute pain when converting from other oral opioids to Opana or Opana ER
Adults: Refer to published relative potency information, keeping in mind that conversion ratios are only approximate. In general, it's safest to start Opana therapy by administering half of calculated total daily dose of Opana in four to six equally divided doses P.O. q 4 to 6 hours. Or, for patients requiring continuous, around-the-clock opioid treatment for extended period, give Opana ER in two divided doses P.O. q 12 hours. Gradually adjust initial dosage of Opana or Opana ER until adequate pain relief and acceptable adverse effects have been achieved.
➣ Moderate to severe acute pain in opioid-experienced patients when converting from Opana to Opana ER
Adults: Administer half patient's total daily oral Opana dose as Opana ER P.O. q 12 hours.
Dosage adjustments
• Mild hepatic impairment (Opana, Opana ER)
• Severe hepatic impairment (Numorphan)
• Moderate to severe renal impairment (Opana, Opana ER)
• Concurrent use of other CNS depressants (Opana, Opana ER)
• Elderly or debilitated patients
Contraindications
• Hypersensitivity to drug or its components, or morphine analogs
• Any situation in which opioids are contraindicated, such as respiratory depression (in absence of resuscitative equipment or in unmonitored settings) and acute or severe bronchial asthma or hypercarbia
• Pulmonary edema secondary to chemical respiratory irritant (Numorphan)
• Suspected or existing paralytic ileus
• Moderate and severe hepatic impairment (Opana, Opana ER)
Precautions
Use cautiously in:
• head trauma, increased intracranial pressure, severe renal disease, hypothyroidism, adrenal insufficiency, urethral stricture, undiagnosed abdominal pain or prostatic hyperpla-sia, biliary tract disease, pancreatitis, extensive burns, alcoholism
• history of substance abuse
• prolonged or high-dose therapy
• elderly or debilitated patients
• labor and delivery
• pregnant or breastfeeding patients
• Pain in immediate postoperative period (first 12 to 24 hours), or if pain is mild or not expected to persist for extended period (Opana ER)
• Children younger than age 18.
Administration
• Give oral on empty stomach at least 1 hour before or 2 hours after eating.
• Tell patient to swallow extended-release tablets whole and not to break, chew, dissolve, or crush tablets.
• Be aware that extended-release tablets are not for p.r.n. use.
• Be aware that extended-release tablets are indicated only for postoperative use if patient had already been receiving drug before surgery or if postoperative pain is expected to be moderate or severe and persist for extended period.
☞ Be aware that administration from any source (such as beverages or drugs) may result in increased plasma drug levels and potentially fatal overdose of oxymorphone.
☞ Keep naloxone available to reverse respiratory depression, if necessary.
• Give I.V. dose by direct injection over 2 to 3 minutes.
Adverse reactions
CNS: somnolence (Opana, Opana ER), sedation, headache, drowsiness, confusion, dysphoria, euphoria, dizziness, hallucinations, lethargy, impaired mental and physical performance, depression, restlessness, insomnia, paradoxical stimulation, seizures
CV: hypotension, orthostatic hypotension, palpitations, bradycardia, tachycardia
EENT: blurred vision, miosis, diplopia, visual disturbances, tinnitus
GI: abdominal distention, flatulence (Opana), abdominal pain, dyspepsia (Opana ER), nausea, vomiting, constipation, biliary tract spasm, cramps, dry mouth, anorexia, paralytic ileus, toxic megacolon
GU: urinary hesitancy or retention, urethral spasm, antidiuretic effect
Respiratory: suppressed cough reflex, hypoxia (Opana), atelectasis, respiratory depression, allergic bronchospastic reaction, allergic laryngeal edema or laryngospasm, apnea
Skin: rash, urticaria, pruritus, facial flushing, diaphoresis
Other: pyrexia (Opana, Opana ER), physical or psychological drug dependence, drug tolerance, allergic reaction, injection site reaction (Numorphan)
Interactions
Drug-drug. Agonist/antagonist analgesia (such as buprenorphine, butorphanol, nalbuphine, or pentazocine): reduced oxymorphone effect; may precipitate withdrawal symptoms (Opana, Opana ER)
Anticholinergics: increased risk of urinary retention or severe constipation
Antihistamines (first-generation), antipsychotics, barbiturates, general anesthetics, MAO inhibitors, sedative-hypnotics, skeletal muscle relaxants, tricyclic antidepressants: increased risk of respiratory depression
Propofol: increased risk of bradycardia (Numorphan)
Drug-diagnostic tests. Amylase, lipase: increased levels
Drug-behaviors. Alcohol use or abuse, opiate abuse: increased risk of respiratory depression
Patient monitoring
☞ Closely monitor respiratory status. Stay alert for respiratory depression and allergic responses affecting bronchi and larynx.
• Monitor vital signs and ECG.
• With prolonged use, watch for signs and symptoms of drug dependence.
• Assess neurologic status carefully. Institute protective measures as needed.
• Monitor patient receiving Opana or Opana ER for breakthrough pain and adverse reaction (especially severe constipation).
Patient teaching
☞ Instruct patient to immediately report seizures or difficulty breathing.
• Tell patient to rise slowly when changing position, to avoid dizziness from blood pressure decrease.
• Instruct patient taking Opana or Opana ER to report episodes of breakthrough pain and adverse reactions (especially severe constipation that may lead to paralytic ileus).
☞ Advise patient not to drink alcohol from any source because doing so may result in fatal overdose.
• Caution patient not to drive or perform other hazardous activities.
• Tell patient not to stop taking drug suddenly after several weeks, because withdrawal symptoms may occur.
• As appropriate, review all other significant and life-threatening adverse reactions and interactions, especially those related to the drugs, tests, and behaviors mentioned above.
oxymorphone
(ox-i-mor-fone) ,Opana
(trade name),Opana ER
(trade name)Classification
Therapeutic: opioid analgesicsPharmacologic: opioid agonists
Indications
Action
Therapeutic effects
Pharmacokinetics
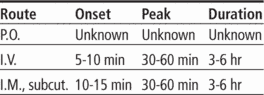
Time/action profile (analgesic effects)
| ROUTE | ONSET | PEAK | DURATION |
|---|---|---|---|
| PO | unknown | unknown | 4–6 hr |
| PO ER | unknown | unknown | 12 hr |
| IM | 10–15 min | 30–90 min | 3–6 hr |
| IV | 5–10 min | 15–30 min | 3–6 hr |
| Subcut | 10–20 min | unknown | 3–4 hr |
Contraindications/Precautions
Adverse Reactions/Side Effects
Central nervous system
- confusion (most frequent)
- sedation (most frequent)
- dizziness
- dysphoria
- euphoria
- floating feeling
- hallucinations
- headache
- unusual dreams
Ear, Eye, Nose, Throat
- blurred vision
- diplopia
- miosis
Respiratory
- respiratory depression (life-threatening)
Cardiovascular
- orthostatic hypotension
Gastrointestinal
- constipation (most frequent)
- dry mouth
- nausea
- vomiting
Genitourinary
- urinary retention
Dermatologic
- flushing
- sweating
Miscellaneous
- physical dependence
- psychological dependence
- tolerance
Interactions
Drug-Drug interaction
Use with caution in patients receiving MAO inhibitors (may result in unpredictable reactions—↓ initial dose of oxymorphone to 25% of usual dose).↑ risk of CNS depression, hypotension and respiratory depression with alcohol, other opioids or CNS depressants including sedatives, hypnotics, general anesthetics, phenothiazines, tranquilizers, skeletal muscle relaxants, or sedating antihistamines ; may initiate therapy with 1/3 to 1/2 usual starting dose.Drugs that may cause volume depletion or hypotension including diuretics, phenothiazines may ↑ risk of severe hypotension.Administration of partial-antagonist opioid analgesics may precipitate withdrawal in physically dependent patients.Nalbuphine, buprenorphine, or pentazocine ↓ analgesia.Concomitant use of kava-kava, valerian, or chamomile can ↑ CNS depression.Route/Dosage
Larger doses may be required during chronic therapyAvailability (generic available)
Nursing implications
Nursing assessment
- Assess type, location, and intensity of pain prior to and 1 hr following IM and 15–30 min (peak) following IV administration. When titrating opioid doses, increases of 25–50% should be administered until there is either a 50% reduction in the patient’s pain rating on a numerical or visual analogue scale or the patient reports satisfactory pain relief. A repeat dose can be safely administered at the time of the peak if previous dose is ineffective and side effects are minimal.
- Patients taking controlled-release tablets should also be given supplemental short-acting opioid doses for breakthrough pain.
- An equianalgesic chart (see ) should be used when changing routes or when changing from one opioid to another.
- Assess BP, pulse, and respirations before and periodically during administration. If respiratory rate is <10/min, assess level of sedation. Physical stimulation may be sufficient to prevent significant hypoventilation. Dose may need to be decreased by 25–50%. Initial drowsiness will diminish with continued use.
- Prolonged use may lead to physical and psychological dependence and tolerance. This should not prevent patient from receiving adequate analgesia. Most patients who receive oxymorphone for pain do not develop psychological dependence. Progressively higher doses may be required to relieve pain with long-term therapy.
- Assess bowel function routinely. Prevention of constipation should be instituted with increased intake of fluids and bulk, and laxatives. Stimulant laxatives should be administered routinely if opioid use exceeds 2–3 days, unless contraindicated.
- Lab Test Considerations: May ↑ plasma amylase and lipase levels. If an opioid antagonist is required to reverse respiratory depression or coma, naloxone is the antidote. Dilute the 0.4-mg ampule of naloxone in 10 mL of 0.9% NaCl and administer 0.5 mL (0.02 mg) by direct IV push every 2 min. For children and patients weighing <40 kg, dilute 0.1 mg of naloxone in 10 mL of 0.9% NaCl for a concentration of 10 mcg/mL and administer 0.5 mcg/kg every 2 min. Titrate dose to avoid withdrawal, seizures, and severe pain.
Potential Nursing Diagnoses
Acute pain (Indications)Chronic pain (Indications)
Risk for injury (Side Effects)
Implementation
- high alert: Accidental overdose of opioid analgesics has resulted in fatalities. Before administering, clarify all ambiguous orders; have second practitioner independently check original order, dose calculations, and infusion pump settings.
- Explain therapeutic value of medication prior to administration to enhance the analgesic effect.
- Regularly administered doses may be more effective than prn administration. Analgesic is more effective if given before pain becomes severe.
- Coadministration with nonopioid analgesics may have additive analgesic effects and may permit lower doses.
- Medication should be discontinued gradually after long-term use to prevent withdrawal symptoms.
- Oral: Administer at least 1 hr prior to or 2 hr after eating.
- Extended Release: Swallow controlled-release tablets whole; do not break, crush, or chew. Titrate to mild to no pain with regular use of no more than 2 doses of supplemental analgesia (rescue) per 24 hr. Dose should be based on 24-hr opioid requirement determined with short-acting opioids then converted to controlled-release form.
- If patient is opioid-naive, start with 5 mg every 12 hr, then titrate in increments of 5–10 mg every 12 hr every 3–7 days to a level that provides adequate analgesia with minimal side effects.
- If converting from Opana to Opana ER, administer half the patient's total daily dose of Opana as Opana ER every 12 hr.
- If converting from parenteral oxymorphone, administer 10 times the patient's total daily parenteral oxymorphone dose as Opana ER in two equally divided doses every 12 hr.
- If converting from other opioids, 10 mg of oral oxymorphone is equianalgesic to hydrocodone 20 mg, oxycodone 20 mg, methadone 20 mg, and morphine 30 mg orally.
Intravenous Administration
- Administer undiluted. Concentration: 1 mg/mL.
- Rate: Give over 2–3 min
Patient/Family Teaching
- Instruct patient on how and when to ask for pain medication.
- Instruct patient to take oxymorphone as directed and not to adjust dose without consulting health care professional. Take missed doses as soon as possible if on chronic therapy. If almost time for next dose, skip dose and return to regular schedule. Do not double doses unless advised by health care professional. Do not stop taking oxymorphone abruptly, may cause withdrawal symptoms. Discontinue gradually under supervision of health care professional. Caution patient to keep medication out of reach of children and pets.
- Advise patient that oxymorphone is a drug with known abuse potential. Protect it from theft, and never give to anyone other than the individual for whom it was prescribed.
- Caution patient not to share this medication; may cause harm or death and is against the law.
- Medication may cause drowsiness or dizziness. Advise patient to call for assistance when ambulating or smoking. Caution patient to avoid driving and other activities requiring alertness until response to medication is known.
- Advise patient to make position changes slowly to minimize orthostatic hypotension.
- Advise patient to avoid concurrent use of alcohol or other CNS depressants with this medication.
- Advise patient to notify health care professional of all Rx or OTC medications, vitamins, or herbal products being taken and to consult with health care professional before taking other medications.
- Encourage patient to turn, cough, and breathe deeply every 2 hr to prevent atelectasis.
- Inform patients taking Opana ER tablets that soft mass resembling tablets may appear in stool; active medication was already absorbed.
- Advise patient to notify health care professional if pregnancy is planned or suspected, or if breast feeding.
Evaluation/Desired Outcomes
- Decrease in severity of pain without a significant alteration in level of consciousness or respiratory status.
Opana
(ō-păn′ə)Latest Searches:
Voraxaze - Voranil - Voorhoeve - voodoo - VOO - Vontrol - von - vomitus - vomiturition - vomitory - vomitoria - vomito - vomitive - vomiting - vomit - vomica - vomerovaginalis - vomerovaginal - vomerorostralis - vomerorostral -
- Service manuals - MBI Corp